Parkinson's disease is characterized by the degeneration of dopaminergic neurons, i.e. neurons that produce the neurotransmitter dopamine. If the level of dopamine in the synaptic cleft between two dopaminergic neurons decreases, this disrupts the associated networks of neurons are disrupted. The University of Tours has developed LBT-999, a tracer with significant affinity and selectivity for dopamine at the synaptic clefts.
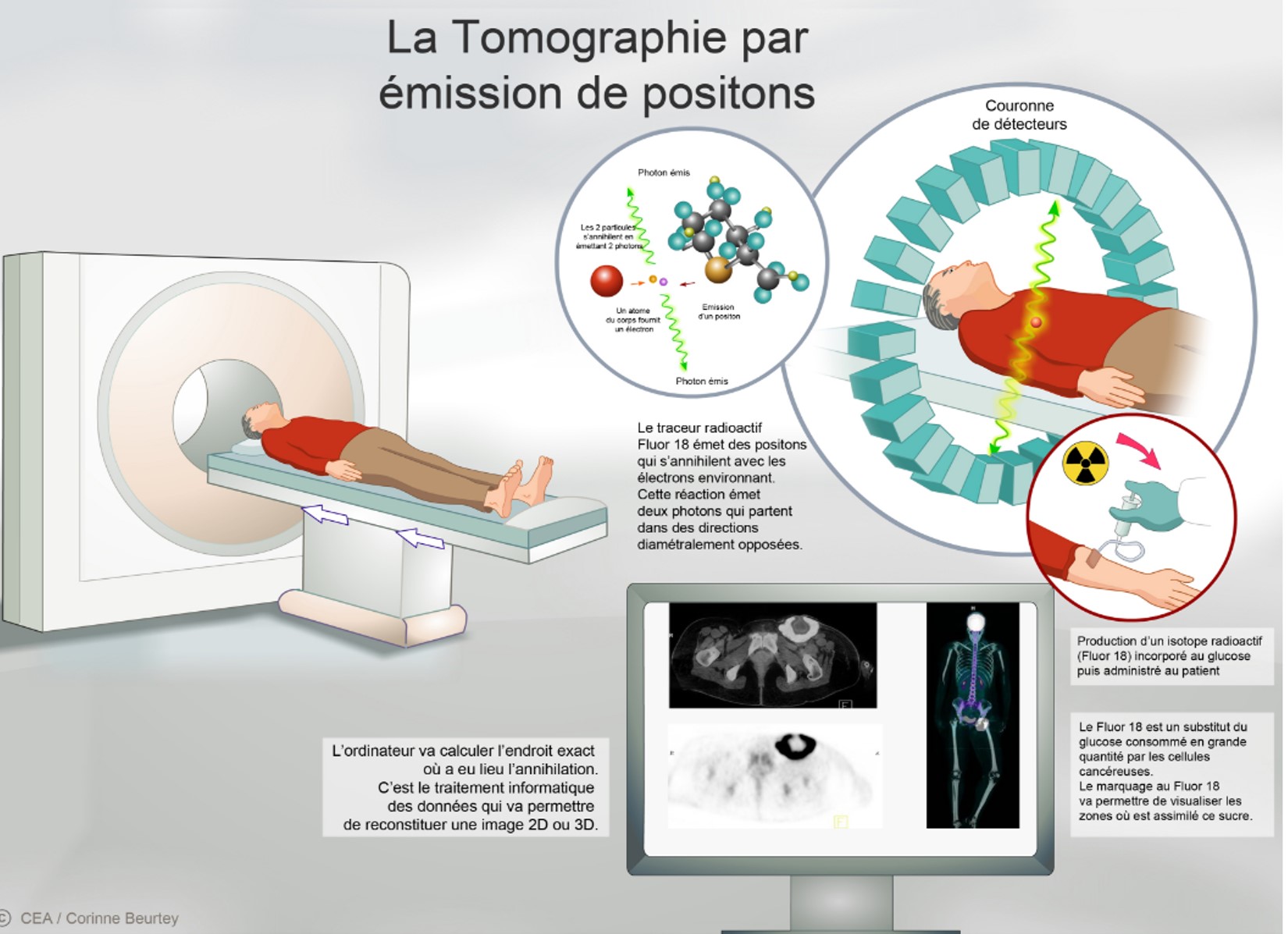
In partnership with Orphachem and the University of Tours, CEA-I2BM has developed an ultrafast technique for the fluorine-18 labeling of this radio-tracer. The process is protected by the filing of a European patent that has recently been transferred to company Cyclopharma under an exclusive licensing agreement for developing a radiopharmaceutical for the differential diagnosis of Parkinson's disease with PET1. Fluoride-18 is a short-lived positron emitter with a half-life of 110 minutes. The fluorine-18-labeled radiotracer, [18F] LBT-999, makes it possible to visualize and quantify the dopamine transporter using PET.
This review could confirm, in a non-invasive way, the clinical diagnosis of Parkinson's disease, and legitimize differentiation from other diseases with similar clinical motor symptoms, such as certain dementias (Lewy Body Dementia). It would make it possible to select the appropriate therapeutics for the patient as early as possible.
The signing of this agreement will lead to the establishment of a Phase III clinical study in early 2017—a crucial step in the marketing process. This follows Phase I currently being carried out at the Tours University Hospital.
The agreement has been covered in a press release
© CEA/ Corinne Beurtey