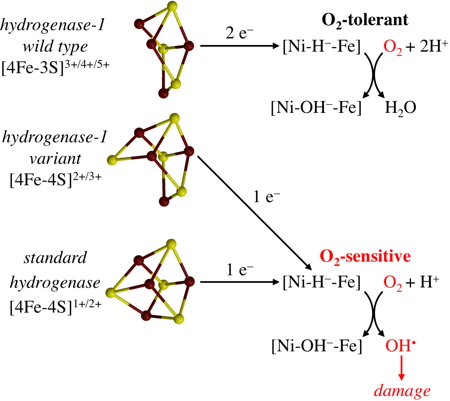
Hydrogenases are enzymes of considerable interest for their potential biotechnological applications both as catalysts in biofuel cells and hydrogen producers. However, these applications can be greatly affected by their reactions with atmospheric oxygen.
In order to better understand this problem, researchers at the IBS investigated a single mutation of the naturally O
2-tolerant
E. coli [NiFe] hydrogenase-1, which makes it O
2-sensitive by changing its [4Fe-3S] cluster into a novel [4Fe-4S] cluster. Their theoretical study explains in detail the observed different redox properties of these two clusters and sheds considerable light on the biological solution to prevent O
2-based deactivation.