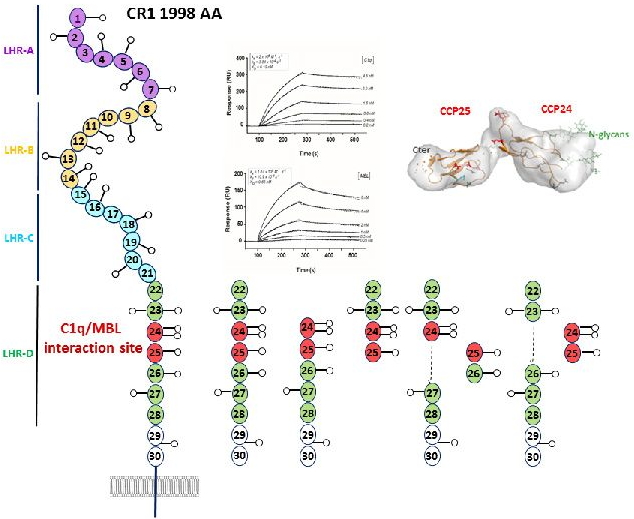
Complement receptor type 1 (CR1) is a multi modular membrane receptor involved in the clearance of complement opsonized components from the blood stream. By binding targets tagged with complement-opsonins, the CR1 receptor on the surface of erythrocytes contributes to their elimination by transport to the liver, then phagocytosis by monocytes, macrophages or neutrophils.
CR1 is composed of 30 homologous complement control protein (CCP) modules and is a receptor for complement-opsonins C3b and C4b. While C3b and C4b have long been known as ligands of CR1, it is only recently that defense collagens such as mannose-binding lectin (MBL), ficolin-2, and C1q have also been shown to act as opsonins. In this study, the IRPAS group located the attachment site of C1q and MBL to CR1 thanks to a molecular dissection strategy particularly adapted to the study of multi-modular proteins. The interaction site for both MBL and C1q is located on the same pair of modules CCP24-25 out of the 30 modules in CR1. This study contributes to enlarge knowledge on the multifunctional role of complement defense collagens and more especially on this new opsonin function that allows the transfer of foreign agents or altereded self on various receptors for clearance.