It has been demonstrated for several years that liquid-liquid phase separation, induced by weak interactions often involving
intrinsically disordered proteins or RNA, provides a very effective means of spatially and temporally controlling cellular processes. The physicochemical properties of these microenvironments provide optimal conditions for enhancing specific molecular interactions, not only by protecting molecules from their environment, but also obviating the need to form membranes. The molecular basis for stabilizing these microenvironments remains poorly understood. IRIG researchers have characterized how these processes are used by measles virus proteins.
Many viruses form compartments in the cells they infect. In this study, researchers at our institute show that when measles virus nucleo-proteins (N) and phospho-proteins (P) are mixed
in vitro, they form membraneless liquid organelles, like oil droplets in water. To understand this process, they use nuclear magnetic resonance (NMR) to identify weak interactions involving intrinsically disordered domains of N and P and show that one of these interactions are essential for phase separation. The researchers follow the modulation of N and P dynamics by fluorescence during droplet formation, and study the thermodynamics of this process by NMR.
They thus observe that when RNA is added to a suspension of droplets formed by MeV N and P proteins, it localizes to the droplets where it induces an
essential step in the virus replication cycle: the assembly of viral nucleocapsids. They also measure that the rate at which this process takes place in these droplets is higher than in the dilute phase. As a result of this work, they are then able to rationalize why viruses form these droplets in infected cells, creating truly ephemeral and transient factories, where they can synthesize nucleocapsids at high speed to ensure their replication, all in a favourable and protected environment.
This study shows that viral replication could be reduced by inhibiting droplet formation, and that the formation of these phases could represent a novel pharmacological target for these viruses.
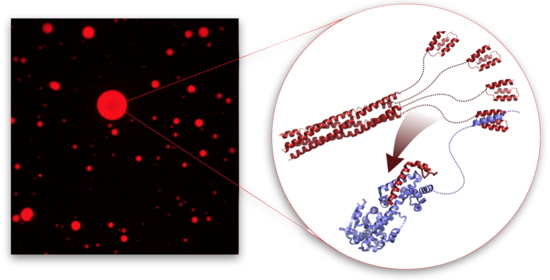
Fluorescence and nuclear magnetic resonance characterization of membraneless droplets formed by measles virus replication proteins.
Intrinsically disordered proteins lack a stable three-dimensional structure and are functional in their disordered state. Their high flexibility allows them to adapt easily to the surface of their partners and are able to fold up during interaction.
This
essential step is the assembly of N protomers into nucleocapsid particles that encapsulate RNA molecules.