An Anglo-French team (AP-HP, Inserm, UPEC, CEA/Mircen, Oxford Biomedica, and
Cambridge University) conducted a phase I/II¹ clinical gene therapy study in
patients with an advanced form of Parkinson’s disease. Fifteen patients were
able to benefit from this treatment that consists of injecting a vector
expressing the genes of three enzymes essential to dopamine biosynthesis (which
is missing in Parkinson’s disease).
Thanks to this therapy, certain cells in the brain begin again to produce and
secrete dopamine. In all patients, the motor symptoms of the disease showed
improvement up to 12 months after treatment delivery. Looking back on the last 4
years, this study demonstrates at this point the safety and tolerability of the
lentiviral vector used for the first time in humans: ProSavin®, developed from
the equine infectious anemia virus (EIAV).
This study was coordinated by Professor Stéphane Palfi, head of the neurosurgery
department at the hôpital Henri-Mondor (AP-HP), within the neurolocomotor center
led by Professor Pierre Cesaro. The CEA’s contribution involved the two
following biomedical platforms:
- MIRCen, a translational research infrastructure
dedicated to biotherapy and imagery in the field of neurodegenerative diseases;
- the Service Hospitalier Frédéric Joliot, which develops functional and molecular
imaging using positron emission tomography, mainly for applications in
neurology, oncology, psychiatry.
This clinical trial follows a preclinical study published in 2009, which showed
for the first time the effectiveness and safety of this drug in an animal model.
Performed within the MIRCen translational platform at the CEA, it opened the way
for the ProSavin® clinical study.
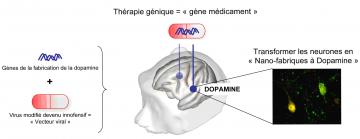
Principe du traitement thérapeutique via un « gêne médicament » (© CEA)
Key figures:
15 patients treated
1 lentiviral vector, used for the first time in humans
3 dose levels tested
Zoom : Parkinson's disease
With
approximately 120,000 patients in France, Parkinson’s disease is the
most common neurodegenerative disorder after Alzheimer’s disease. It is
expressed mainly through motor symptoms of progressive and increasing
severity, such as tremors, rigidity of the limbs and reduced body
movements. This condition is due to the degeneration of neurons that
produce dopamine, a neurotransmitter involved in motor control.
Currently, the treatment of people with this disease consists of taking
drugs that mimic the action of the missing dopamine in the brains of
these patients. If this treatment provides a good improvement in motor
activity in the early stages of the disease, severe side effects occur
over time, including fluctuations in the treatment effect and
involuntary movements (dyskinesia).
[1] Tested for the first time in humans with three levels of increasing doses (1x,
2x and 5x).