Research projects of Marie-Pierre Heck
Synthesis and study of new cucurbiturils

Cucurbiturils, CB[n], are synthetic crown-shaped oligomers with a hydrophobic cavity accessible by two carbonyl portals that confer them properties of supramolecular recognition of "host-guest" type.
Despite the importance of gases in environment, science and medicine, their molecular recognition, especially in water, is not well documented. Interested in the encapsulation of rare gases and small alkanes in water, we have designed and synthesized a water-soluble cucurbiturils family, called CynCB[6] which have cyclohexyl units (n = 1-6). We have shown that the presence of one cyclohexyl group is sufficient to confer to these macrocycles a good solubility in water. After functionalization, they should be particularly interesting as xenon biosensors, able to image tissues and organs.
New unsaturated bambusurils
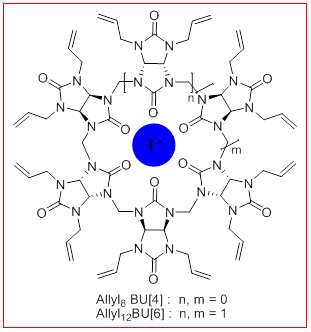
Bambusurils,BU[n], is a new family of synthetic macrocyclic whose first members were discovered in 2010. They differ from the CB[n] by their functionalized glycoluril units giving them a more flexible alternated structure and an internal cavity able to encapsulate anions. The latter, especially iodides, play important roles in biology and medicine. Having specific receptors is necessary to follow the path of anions, to measure and/or to extract them, and to understand their coordination. New bambusurils, allyl8BU[4] and allyl12BU[6] bearing alkenes, have been prepared by a new method using microwaves. They can be functionalized by cross-metathesis reaction, and important applications of these bambusurils as multivalent platforms of interest for complexation of iodides in the medical field can be envisaged.
Development of metathesis catalysts
As a part of a collaborative European project called Metacode (Code-engineered new-to-nature microbial cell factories for novel and safety-enhanced bio-production), the metathesis reaction is studied to be used in biological media to allow its use as an orthogonal reaction in synthetic biology.