A collaborative international project involving researchers from CEA (French Atomic Energy Commission), CNRS (French National Centre for Scientific Research), Inserm (French National Institute of Health and Medical Research), University Grenoble-Alps, University of Montpellier and Stanford University[1] has identified the enzyme, Tubulin CarboxyPeptidase (TCP), responsible for the biochemical transformation of cellular microtubules, through detyrosination. Detyrosination is a biological reaction resulting in the removal of the terminal amino acid tyrosine[2] from α-tubulin, one of the subunits making up microtubules. After four decades of research, biologists have finally succeeded in purifying this protein, and have gone on to provide evidence of its cellular activity.
Microtubules contribute to essential cellular functions
Microtubules are dynamic fibres present in all cells, they are formed by the combination of two proteins (α-tubulin and β-tubulin). Microtubules fulfil numerous functions: they separate the chromosomes to distribute them between the two daughter cells during cell division; they contribute to cellular polarity, morphology and to cell migration; they also form a network inside the cell, on which cellular constituents, such as proteins or RNA strands, can be transported.
These cellular functions are regulated by "signals" which are present on the surface of microtubules. These signals, consisting of biochemical modifications to amino acids (known as post-translational modifications, as they take place after protein synthesis), occur at multiple sites in cells and are executed by a broad range of enzymes; in this case, the enzymes modify the tubulins.
The enzyme TCP, finally identified after 40 years of mystery
The activity of one of these enzymes was first identified in 1977 by an Argentinian research team. They named this activity "TCP" (Tubulin CarboxyPeptidase). This enzyme was shown to perform the detyrosination reaction: removal of the terminal amino acid, a tyrosine, from the end of α-tubulin, but the protein itself remained to be identified (its size and sequence were unknown). Another enzyme, the ligase TTL, performs the reverse reaction – tyrosination – through which the tyrosine is replaced. The detyrosination/tyrosination cycle is vital for the cell and the organism as a whole. Massive (abnormal) detyrosination is observed in a number of severe cancer types and in cardiovascular diseases.
The identification and characterisation of TCP was therefore a major objective for researchers wishing to understand the physiological function of α-tubulin detyrosination and to determine the consequences of its inhibition.
To isolate TCP, the researchers monitored its activity, used conventional biochemical techniques, and collaborated with chemists from Stanford University, who developed a small molecule inhibiting its activity. This molecule was then used as bait to "trap" the elusive enzyme.
Tubulin detyrosination/tyrosination cycle
Microtubules are fibres which are present in all cells, comprised of a stack of α/β tubulins. α-tubulin has a tyrosine (Y) at its extremity, which is alternately removed and replaced by two enzymes, thereby modifying the microtubule’s surface. TCP (represented by a saw composed of two elements, VASH/SVBP) is responsible for detyrosination. TTL (represented by a tube of glue) replaces tyrosine on the tubulin. This cycle is essential to the various roles played by microtubules in cells (division, migration, etc.) and is vital for the organism as a whole. © C. Bosc, GIN
|
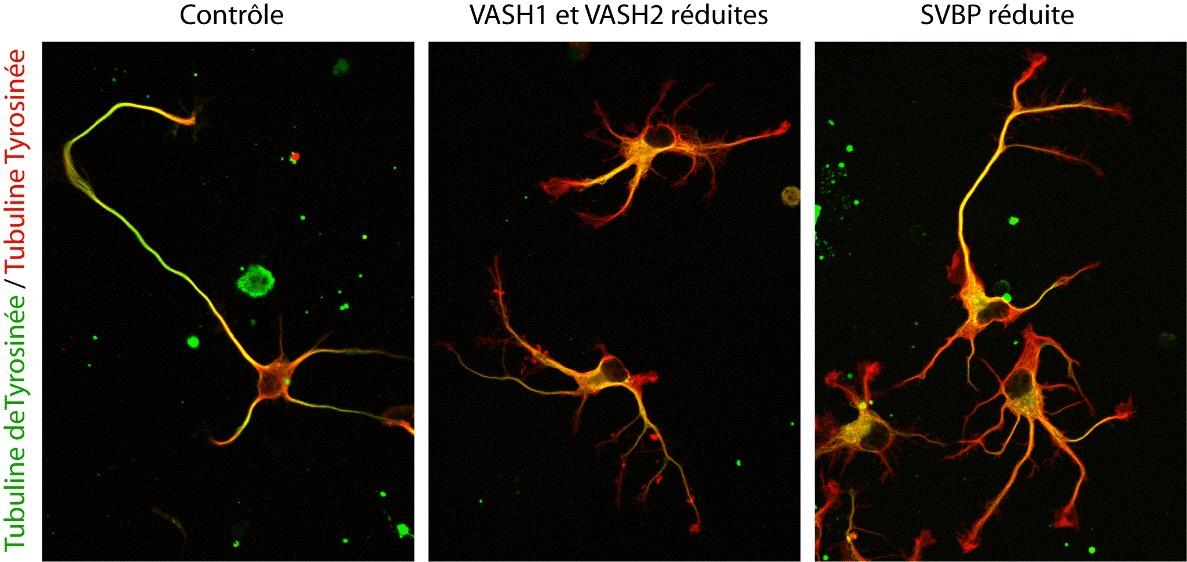
In the end, not one, but two enzymes were discovered. VASH1 and VASH2 were already known to scientists, but they had not previously been associated with the cytoskeleton. The researchers demonstrated that, when associated with a partner protein called SVBP, VASH1 and VASH2 can cause detyrosination of α-tubulin. To demonstrate this activity, the team inhibited the expression of VASH1/2 (or their partner SVBP) in neurons. In both cases, a very strong decline in the level of detyrosination of α-tubulin was observed along with altered neuronal morphology (see Figure). The researchers went on to demonstrate that these enzymes are also involved in the development of the cerebral cortex.
Prospects for the fight against cancer
Thus, forty years after the first evidence of α-tubulin detyrosination was presented, the enzymes responsible have been identified. Scientists are now hoping that, by modulating TCP activity and improving their knowledge of the detyrosination/tyrosination cycle, they will be able to develop new treatments for specific cancers, and to gain a better understanding of its cerebral and cardiac functions.
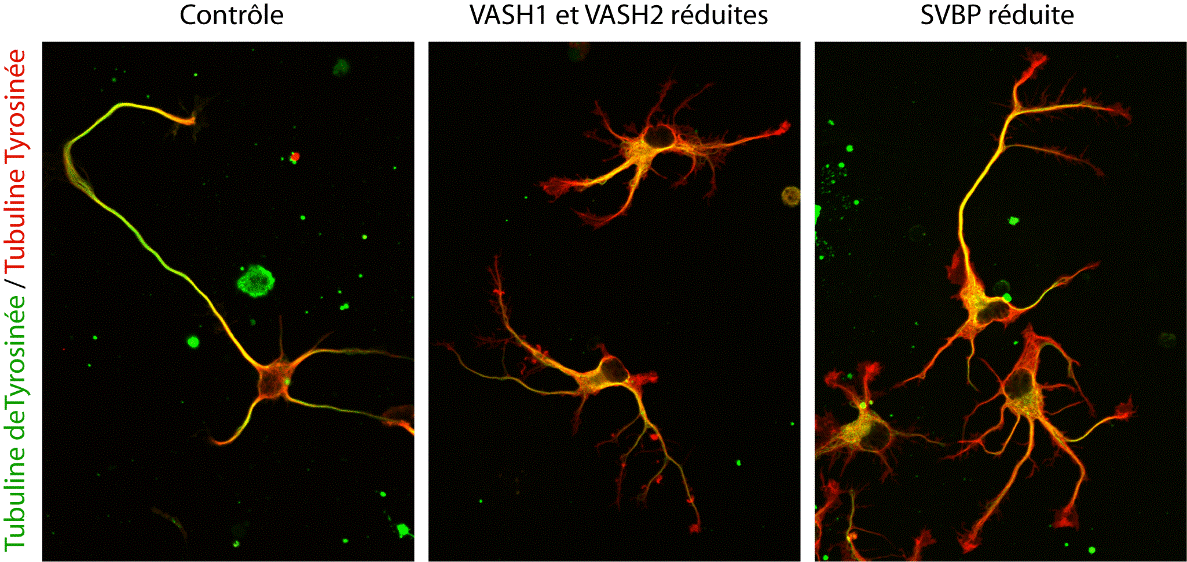
Photographs showing alterations to neurons as a result of reduced expression of TCP enzymes (VASH/SVBP). From left to right: control neuron, neurons expressing reduced levels of VASH1 and VASH2, neurons expressing reduced SVBP levels. Development is delayed in neurons with reduced enzyme levels, and morphological anomalies are seen.
© L. Peris /GIN
[1] The following institutes were involved: Grenoble Institute of Neurosciences, GIN (Inserm/Univ. Grenoble-Alps); Institute of Biosciences and Biotechnologies of Grenoble, BIG (Inserm/CEA/Univ. Grenoble-Alps); Institute for Advanced Biosciences, IAB (Inserm/CNRS/Univ. Grenoble-Alps), Department of Pathology, Stanford University School of Medicine (Stanford, USA), Institute of Human Genetics, IGH (CNRS/Univ. of Montpellier), Centre for Biochemical and Macromolecular Research Montpellier, CRBM (CNRS/Univ. of Montpellier).
[2] Tyrosine is one of the 22 amino acids making up proteins.