The Biocapan (BIOactive implantable Capsule) European research project, coordinated by CEA-
Leti, is helping to develop innovative cell therapies. The teams assigned to this research program are developing a novel therapeutic drug to treat type 1 diabetes. Their approach uses biocapsules, into which pancreatic cells are inserted; these cells synthesize insulin, a substance in which diabetic patients are deficient. These biocapsules facilitate transplants of such cells by protecting them against any immune response. The capsule is permeable to nutriments and other elements (such as oxygen) that the pancreatic cells need in order to survive, but is impermeable to antibodies and other immune cells that might otherwise seek to eradicate them. As a result, the cells continue to function; the insulin that they produce is secreted from the capsule, helping to palliate the poor function of the patient’s pancreas.
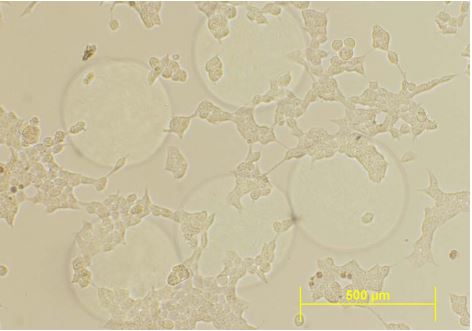
200 uM Microcapsules with epithelial cells HEK-293 in culture. © NIT
How does BIOCAPAN enhance the lives of diabetics?
Patients suffering from type 1 diabetes must constantly monitor their blood sugar level. Additionally, they must inject insulin to compensate for inadequate production by their pancreas. Some patients need such injections multiple times daily. One of the treatment options currently being researched consists in transplanting insulin-secreting cell clusters known as Langerhans islets; clinical trials are underway but this treatment requires lifelong administration of high doses of immunosuppressants.
The BIOCAPAN project aims to develop this innovative cell therapy in order to eliminate the need to inject insulin or take immunosuppressive drugs. It is aimed at patients with type I diabetes and one of the six forms of type II diabetes, representing a worldwide target population of around 80 million people. The biocapsule, made from a number of polymers, serves two purposes: preventing the patient’s body from rejecting the transplanted cells, and helping the transplanted cells to perform their insulin-secreting function. Using this therapy should make it possible to reduce or eliminate the need for heavy immunosuppressive treatments, which often have multiple side-effects.
This project aims to finalize development of this biocapsule, define a production system to enable the drug to be manufactured and then validate the results in pre-clinical trials, before potentially moving on to the various stages of the marketing authorization process.
The treatment must therefore satisfy a number of criteria. The team must resolve a combination of logistics-related and regulatory problems. With this type of treatment, a range of factors must be reliably defined, including the injected dose, which in this case will depend on a combination of the number of cells in each capsule (determining the quantity of insulin produced by the biocapsule) and the number of biocapsules administered in the injection. Marketing authorization will not be granted unless this data is perfectly standardized. CEA-Leti is particularly active in the area of developing automated tools to enable standardized, reproducible production of this type of cell therapy.
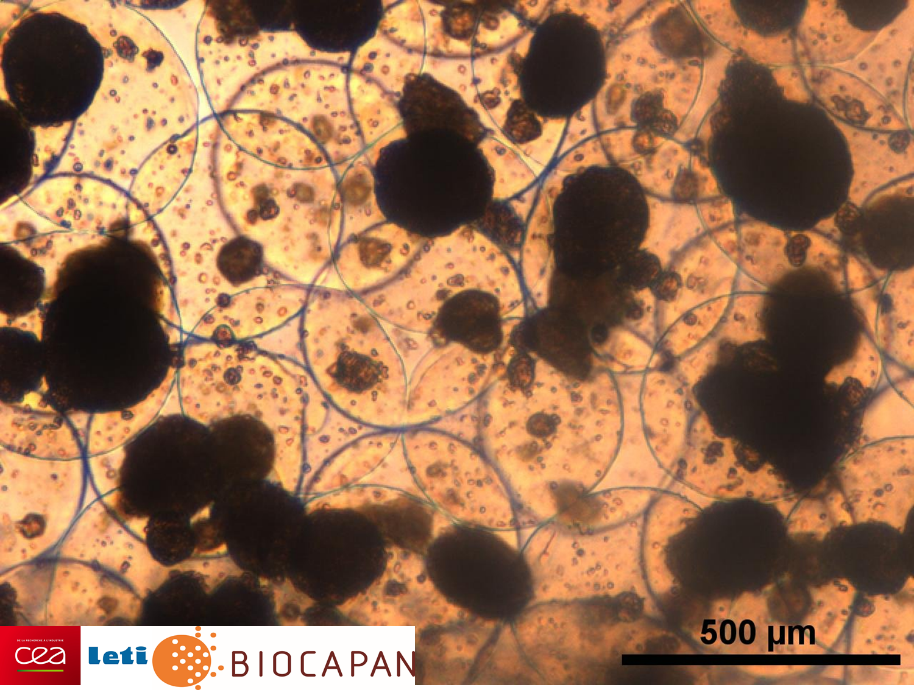
Encapsulated human pancreatic islets. © CEA-Leti
About BIOCAPAN

With 8 M€ in EU funding from 2015 to 2019 and under the coordination of CEA, the nine members (from six countries) of the BIOCAPAN project aim to develop an innovative treatment for Diabetes Mellitus. Based on the implantation of smartly microencapsulated allogeneic islet cells, it will allow an effective long-lasting blood glucose normalization and stabilization, without the need for immunosuppressants.