Inflammatory bowel diseases (IBD) are the second most common immune-mediated disorders in Europe, affecting more particularly young people. The current therapies, including antibodies, show three main drawbacks: efficacy, tolerability and convenience. If successful, New Deal solution will offer radical therapeutic progress for all IBD patients, thanks to the improved efficacy and increased safety of the specific JAK31 inhibition. In clinics, this agent has proven to be a target of great interest, with a better tolerability of siRNA2 in term of immunogenicity and a good convenience with oral administration.
3 million Europeans suffering
Inflammatory bowel diseases affect more than 3 million people in Europe. Among these IBDs, Crohn’s disease and ulcerative colitis, which are chronic and immune-mediated disorders of the gastrointestinal tract, affect mainly adolescents and young adults. The patients suffer from symptoms such as diarrhoea or abdominal pain but can also lead to bowel perforation, severe bleeding or cancer. For now, conventional IBD therapies can only treat half of the patients with ulcerative colitis and no patients with Crohn’s disease. Biological inflammatory bowel disease therapy can only treat a minority of patients because of immunogenicity and an increasing risk of infection.
To achieve this challenge, the partners will address three objectives:
- Inhibit specifically JAK3 in a highly selective and safer manner by the mean of siRNA and RNAi interferences carefully designed and validated;
- Deliver the siRNA therapeutic locally to the inflamed gut, by combining innovative nanostructured lipid carriers enabling their transport across the mucus, the intestinal barrier and the plasma membrane of the target cells, with smart polymeric capsules for protecting siRNA nanotherapeutics during their transit along the gastro-intestinal tract. Then, the microcapsules will be administrated orally and transit along the gut intestinal tract;
- Promote the clinical translation and the future industrial transfer of this new clinical product, by addressing manufacturing, safety and efficacy evaluation at the late preclinical stage, to generate a regulatory submission package and a clinical development plan.
1 JAK3 (Januse Kinase 3) is a human enzyme, showing promising medical application.
2 SiRNA means ‘small interfering RNA’ and can prevent the expression of some genes.
3 RNAi stands for ‘RNA interference’. It is a biological process in which RNA molecules inhibit gene expression or translation, by neutralizing targeted mRNA molecules.
4 The CEA-Leti, a technology research institute of the CEA, pioneer in micro and nanotechnologies, and tailoring differentiating applicative solutions that ensure competitiveness in a wide range of markets is coordinating the project. The CEA-BIG, specialized in bioscience and biotechnology, is also involved.

Physico-chemical characterization of drug-loaded lipid nanoparticles in the lab, with Dorothée Jary, WP2 leader in NEW DEAL and Fabrice Navarro, Coordinator of NEW DEAL.
© CEA /L. Godart
The New Deal project brings together clinical experts on IBD, leading scientists in nanomedicine, RNAi3 biology and immunology, innovative SMEs with a strong background in nanosafety, design of capsules and regulatory issues and an established pharma company with historic expertise on gastroenterology medicinal products. If successful, New Deal will open new avenues for siRNA-based therapy in IBD with local administration. “The New Deal concept could revolutionise IBD therapies for the great benefit of patients and European healthcare systems”, explains Dr Fabrice Navarro (CEA4), the coordinator of the project.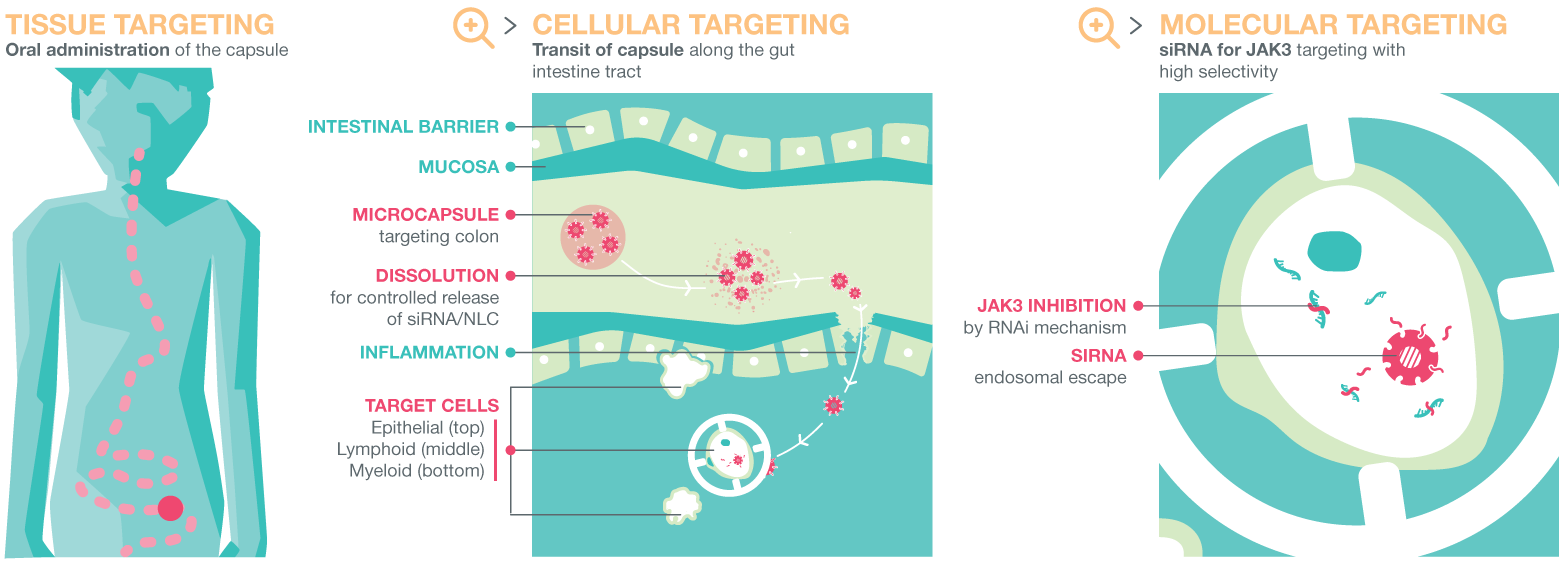
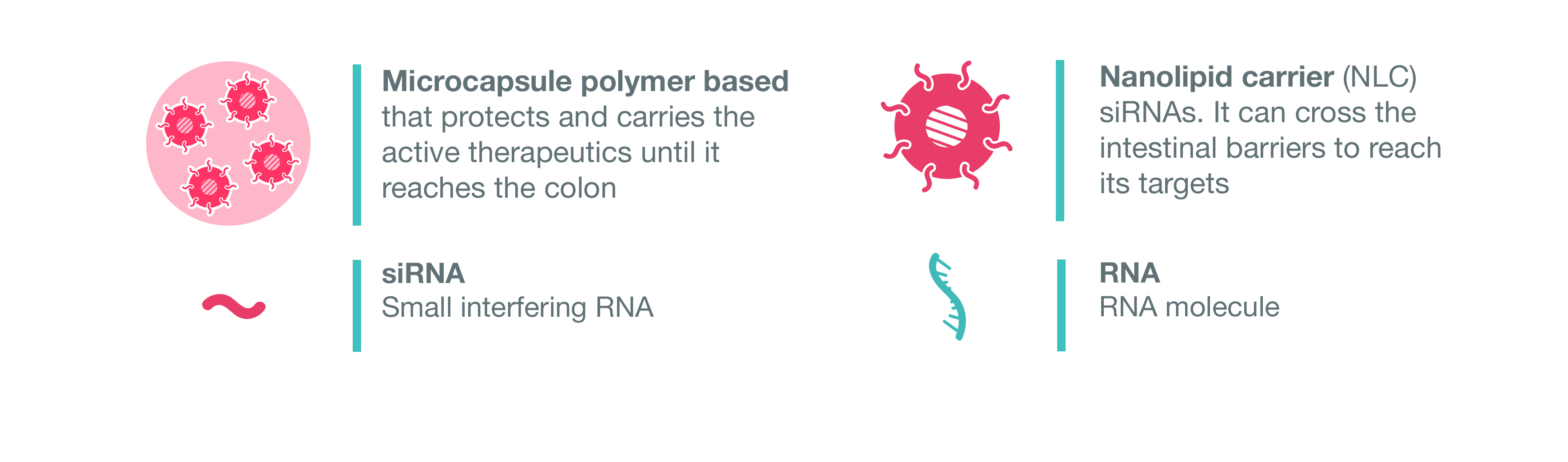
Scheme of NEW DEAL concept. © NEW DEAL Project
About NEW DEAL
Launched in January 2017, INSIDER is bringing together 11 different partners from 5 European countries over a four-year period. Having received EU funding of 6 million euros, the project will offer radical therapeutic progress for all IBD patients and open new avenues for siRNA-based therapy in IBD with oral administration.