Nanomedicine covers all medical applications of nanotechnology and nanomaterials. The regulatory challenge of nanomedicine is the evaluation of the quality, safety and efficacy of nanoscale materials prior to their regulatory approval. As the size of nanomaterials is similar to that of most biological molecules and structures, it can be useful for both in vivo and in vitro biomedical research and applications. As an example, the integration of nanomaterials with biology has already led to the development of diagnostic devices, contrast imaging agents, physical therapy applications, and drug delivery vehicles.

View of the Nano-SafetyPlatform (PNS), located on the CEA’s center of Grenoble. In the PNS, CEA is conducting experimentations for EUNCL. © Denis Morel/CEA
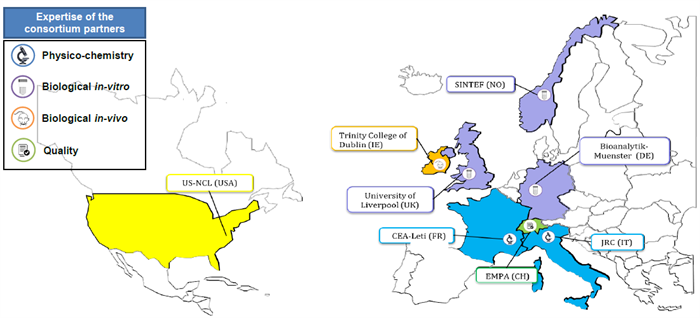
In order to accelerate the development of nanomedicine business in Europe, the main goal of the EUNCL (standing for European Nanomedicine Characterization Laboratory) project, led by CEA, is to support the development of promising medical nanomaterials from the R&D community. Within EUNCL, eight European and one American analytical reference facilities are connected to offer a wide panel of analytical assays. Helping it leads those promising nanomedicines through the so-called ‘valley of death’ in the earlier stages of their preclinical development. Characterising nanomedicines is a prerequisite for researchers to fully evaluate the critical parameters such as biodistribution, metabolism, pharmacokinetics, safety profiles and immunological effects.
A unique European infrastructure to benchmark nanomaterials
The EUNCL infrastructure fosters innovation in nanomedicine by providing access to a trans-disciplinary testing infrastructure, at the state of the art, for a full characterisation of nanomedicines, developed by public labs, spin–offs and innovative SMEs. The infrastructure provides a comprehensive set of characterisation assays (physical, chemical, in vitro and in vivo biological properties) allowing researchers in academia, industry and government to better understand or predict the in vivo effects of their nanomedicines. Full characterisation is also required by regulation agencies before approval of any tests on human beings.
Being the only infrastructure in Europe allowing the complete nanocharacterisation of nanomedicines, the EUNCL has been aligned, in just 18 months, with the standards of the reference infrastructure in the USA (the NCI—NCL) and became its unique European interface as well as the interface for European regulatory authorities.

Formulation of nanoemulsions with different concentrations. © Patrick Avavian/CEA
Increasing the European expertise on nanomaterials used for medical applications and beyond
The knowledge base developed by EUNCL will help the European Medicines Agency (EMA) or other relevant agencies to adapt the current regulation and approval process to nanomedicine products. Analytical assays will be refined or new ones will be developed to take into account the specificities of nanomaterials compared to small molecule drugs. Therefore, the knowledge gathered by EUNCL will be helping a large array of European institutions, not only the ones working on the medicinal field, working for the benefits of European citizens.
External European analytical centres with complementary or highly specific analytical expertise or infrastructures are associated to EUNCL as Satellite Labs.

Real-time process control of drug and vaccine design, at Leti (CEA Tech). © Bruno Amsellem/CEA
Providing services for European companies
EUNCL has a strategic and political role in helping newcomers, like spin-offs or SMEs, in getting an easy access to nanocharacterisation and further to prepare their submission for product regulatory approval. The project provides a single entry point to critical infrastructure and characterisation services to qualify the selected nanoparticles' physical and chemical attributes, their in vitro biological properties, and in vivo compatibility. The EUNCL objective is to support European companies to anticipate the regulatory review of nanotechnologies intended for innovative therapies and diagnostics.
To assist European nanomedicine producers, EUNCL offers a comprehensive portfolio of 40 assays (called Assay Cascade) aimed at evaluating the quality and safety of their nanomaterials. Around a year is required to characterize a nanomaterial from application through the in vivo phase.
Moreover, EUNCL supplies applicants with a complete and detailed characterisation data set that enables researchers in academia, industry, and government to facilitate further development and translation of their nanotechnology strategies towards clinical applications. The EUNCL project accepts continuous application to its call, with two cut off dates in April and October each year. All types of nanomedicines, with putative application for treatment and/or diagnosis via injection or oral formulation, are scrutinized. Any selected project will be provided with the EUNCL’s services at no cost for the submitting sponsor. 18 applications have been reviewed in the first two campaigns, between August 2016 and October 2017.
About EUNCL

With 5 M€ in EU funding from 2015 to 2019 and under the coordination of CEA, the objective of the nine members (from height countries) of the EUNCL project is to reach a level of international excellence in nanomedicine characterization for all medical indication, and make it accessible to all organisations developing candidate nanomedicines prior to their submission to the regulatory agencies in order to get the approval for clinical trials and later the marketing authorization.