The Arrhenius law is widely used to describe and calculate the variation in the rate of a chemical reaction as a function of temperature. According to the law, in a given material at an ultra-low temperature, the motion of any atoms heavier than hydrogen is substantially slowed or even "frozen": it describes the material and its defects as motionless. A study published in Nature Materials on January 27, 2020, now identifies limitations of the Arrhenius law on this point.
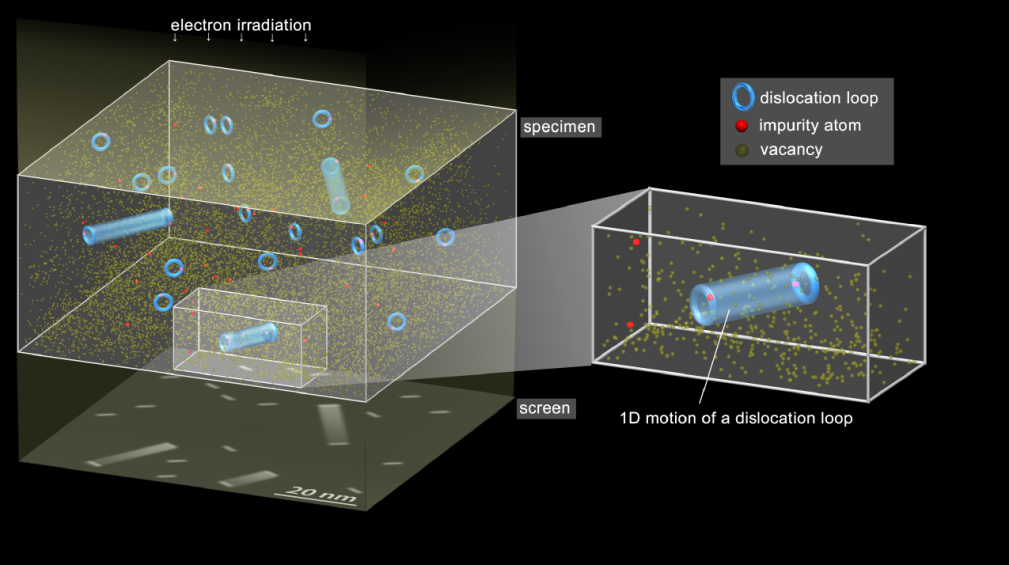
Motion of dislocation loops in low-temperature tungsten
Researchers looked into the motion of defects in a tungsten sample (1) at cryogenic temperature. In a material of this kind, clusters of interstitial atoms (2) form at nanometer scale and are trapped by impurity atoms (see illustration). By subjecting this material to electron irradiation, the researchers observed that a cluster can leave the perimeter of one impurity before being trapped by another.
To interpret their experimental measurements, they had to use quantum mechanics, a theory which explains the phenomena observed when the behavior of particles of very low mass, such as electrons, muons or even hydrogen atoms, is studied. Quantum mechanics is therefore essential to understand, calculate and simulate the evolution of materials and their microstructures.
These results suggest that all the classic experiments conducted at cryogenic temperatures need to be revisited (e.g. annealing experiments to measure variations in material resistivity or internal friction), where quantitative interpretation has to date been based solely on the Arrhenius law.
(1) The mass of a tungsten (W) atom is equivalent to that of 183 hydrogen (H) atoms.
(2) These defects consist of "interstitial" atoms. These are badly positioned extra atoms in the regular stack of atoms constituting the crystal lattice of a metal or mineral.