Drug discovery and development is a time-consuming, expensive and risky process since only one product on ten entering clinical trials will reach the market and this ratio is even reduced when targeting the central nervous system. This dramatic attrition of drug candidates is mainly related to poor efficacy and unexpected adverse effects observed in Phase II clinical trials1. This results in part from insufficiently addressed body distribution studies, accumulation and/or metabolization assessments which all are strongly limited by the lack of chemically labeled compounds available. One solution would be to test a larger number of labeled molecules in preclinical trials, but the number of isotopic labeling methods applicable to drugs candidates remains limited.
Coordinated by the Institute of Biology and Technology (CEA, Saclay, France), ISOTOPICS aims at the development of an innovative isotopic chemistry for the labeling of large series of drugs including chemicals and biologics with (radioactive but not only) isotopes of hydrogen, carbon and fluorine that can be detected and quantified in trace amounts in biological fluids and through whole-body imaging.
This new chemistry is expected to enable the easy and quick identification of new drug candidates and to reject any candidate which might display insufficient efficacy or unpredictable adverse effects before the compound is selected for clinical trials. It is also expected to help in the development of new PET2 tracers for medical diagnostic.
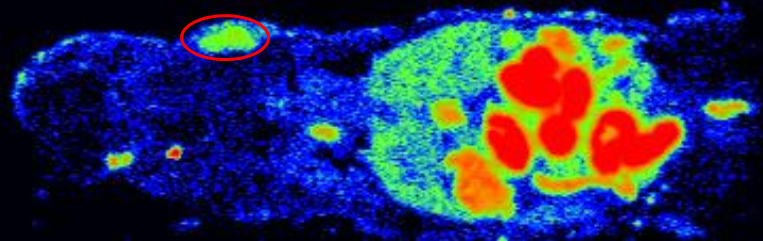
Tomography of a mouse after injection of a radiolabeled drug candidate. © CEA
ISOTOPICS is anticipated to improve Drug Discovery and Development by decreasing drug attrition, streamlining the precocious in vivo evaluation of drug candidates to explore new therapeutic solutions, and training the next generation of (radio)labeling-specialized chemists with a strong background in medicinal chemistry through research lab experience, secondments in other partner’s premises, taught courses and workshops, and complementary skills training.
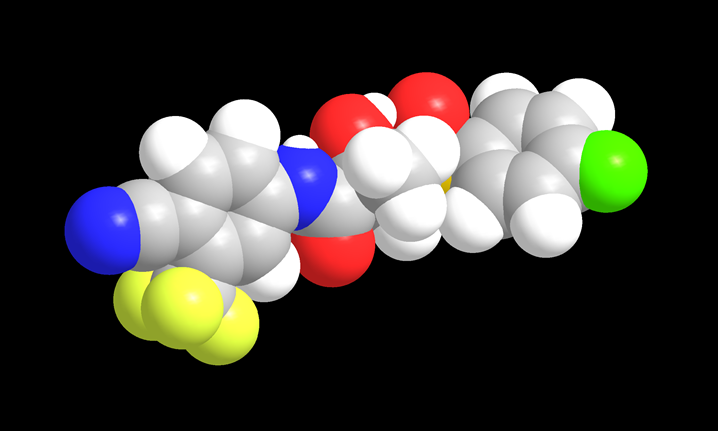
Example of a drug radiolabeled with fluorine-18, a PET emitter (shown in light green). © CEA
1 The phase II of a clinical trial corresponds to the testing of a drug on patients to assess its efficacy and safety. It follows a phase of test on non-human subjects (preclinical) and dose-ranging tests (phase I) and precedes the testing of its effectiveness (phase III) and post marketing surveillance once it is used by physicians (phase IV)
2 PET stands for Positron Emission Tomography which is a nuclear medicine technique that is used to observe many biological processes in the body.
About ISOTOPICS :
|
Gathering 8 partners and benefiting from an EU contribution amounting to 3 948 843.96 €, this highly interdisciplinary project is expected to have a profound beneficial impact on drug innovation in Europe by providing novel efficient techniques and new experts in the fields of labeling and medicinal chemistries.
|