Better arterial healing and a lower
risk of thrombosis after percutaneous neurological and coronary interventions.
Thrombosis is a major complication of surgeries to open up arteries in the brain and heart. AlchiMedics has developed a coating technology named “Electro-grafting”, delivering covalently bonded nanometric polymer brushes, that accelerates healing and reduces thrombosis by accelerating healing.
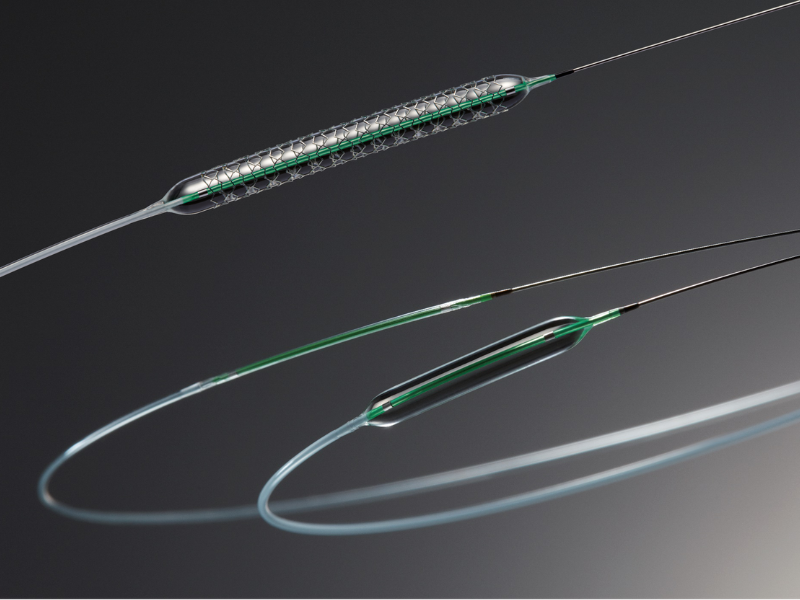
RAVE HT. Credit : SanaVascular
AlchiMedics’ grafted polymer coating accelerates the migration of healing endothelial cells from the inner walls of the arteries while inhibiting the proliferation of smooth muscle cells likely to cause occlusion, accelerating the healing of arteries inevitably wounded after a percutaneous intervention. The polymer is electrografted on to the surface of the medical device in a very thin layer (just 150 nanometers thick).
The risks of acute and late stent thrombosis are virtually eliminated once the endothelium is formed, and Dual Anti-Platelet Therapy (DAPT) can be safely interrupted at 6 months (the Pioneer IV trial in Europe is presently assessing safety with 1 month DAPT). The technology has made brain and heart stent intervention safer for the 1.5 million patients in eleven countries who have already benefited from this technology.
AlchiMedics’ technology is protected by 225 patents, 150 of which belong to the CEA. The company has been commercializing its solution on the Chinese market since 2011 (and has earned CFDA approval), in Europe since 2019 (with the CE marking for its HT Supreme stent), targeting approval in the United States in 2023 (with FDA premarket approval for the RAVE HT stent). AlchiMedics is continuing to improve its coating technologies to bring patients even greater levels of safety and, ultimately, eliminate the need for blood thinners.
Key figure: 2 millions
2 millions patients worldwide have stents coated using AlchiMedics’ technology.
KEY Markets:
- Interventional neurology: active stents and flow diverters for intracranial stenosis and the treatment of aneurysms
- Interventional cardiology: coronary and peripheral stents, heart valves
Technologies used:
- Electrografting polymers that promote healing on implantable medical devices
- A biodegradable polymer for the controlled release of the active ingredient
- Biomimetics
Year founded: 2007
CEA Institute: CEA-Iramis