Mimic photosynthesis of plants to convert, thanks to light, stable and abundant molecules such as water and CO2 into high energy fuel (hydrogen) or chemicals of interest to industry, is today a major challenge of research. The realization of an artificial photosynthesis in solution remains however limited by the use of compounds based on expensive and toxic metals in order to capture light. Researchers from CNRS, CEA and Université Grenoble Alpes offer an efficient alternative with semiconductor nanocrystals (or "quantum dots"), based on copper, indium and sulfur, less expensive metals and less toxic. This work is published in Energy & Environmental Science on april 2018.
© Damien Jouvenot, Department of Molecular Chemistry (CNRS / Université Grenoble Alpes).
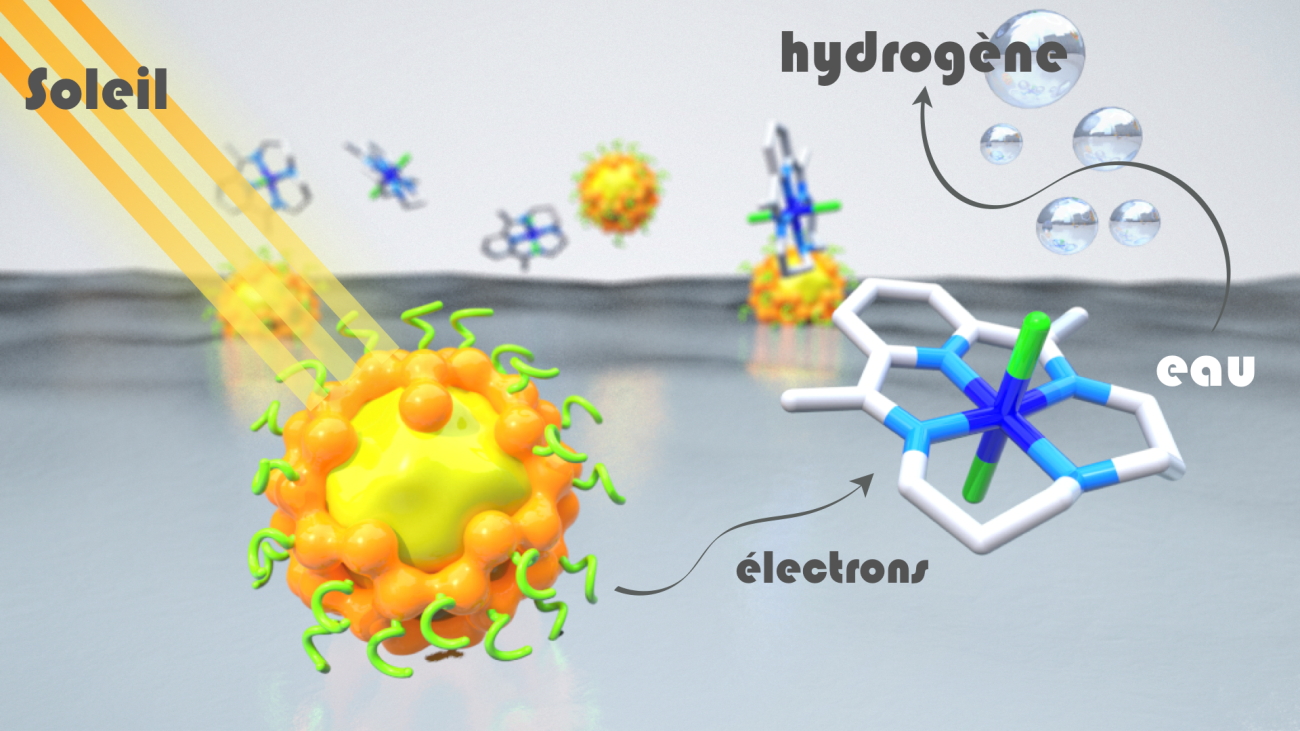
In artificial photosynthesis systems, chromophores, or "photosensitizers", absorb light energy and transfer electrons to the catalyst, which activates the chemical reaction. While many advances have been made in recent years in the development of noble metal-free catalysts, most photosensitizers still rely on molecular compounds based on rare and expensive metals, such as ruthenium or iridium, or inorganic semiconductor materials containing cadmium, a toxic metal.
For the first time, researchers from the Department of Molecular Chemistry (CNRS / Université Grenoble Alpes) and SyMMES (CNRS / CEA / Université Grenoble Alpes) have demonstrated, by combining their expertise in semiconductor materials engineering and photocatalysis, that it is possible to produce dihydrogen very efficiently by combining inorganic nanocrystals (or quantum dots) consisting of a core of copper and indium sulphide, protected by a shell of zinc and sulfur, to a cobalt-based molecular catalyst. This "hybrid" device combines the excellent absorption properties of visible light and the stability of inorganic semiconductors with the efficiency of molecular catalysts. In the presence of an excess of vitamin C, which supplies the electrons to the system, it shows a remarkable catalytic activity in water, i.e. the best obtained to date with "quantum dots" without cadmium. The performance of this system is much better than that obtained with a ruthenium-based photosensitizer, thanks to the very high stability of its inorganic materials, which can be recycled several times without significant loss of activity.
These results highlight the great potential of such hybrid systems for the production of hydrogen from solar energy.
This work was done as part of a collaborative project funded by Labex Arcane Grenoble.