Our cells permanently receive and transmit signals from neighboring cells or specialized tissues, which allows the harmonious development of the organism and ensures organs function all along our life. These signals are, for example, differentiation factors and cell growth or pro-inflammatory molecules that bind to receptors expressed at the cell surface. Once bound to its ligand, the receptor activates one or more intracellular signaling pathways in cascade instructing cytoplasmic or nuclear effectors that induce an appropriate cell response. Signal arrest often depends on the internalization of the receptor by endocytosis and its subsequent degradation by the lysosomal pathway.
Most of the times, the binding of the ubiquitin polypeptide to the plasma membrane receptor is the primary molecular signal that induces its internalization. By specifically hydrolyzing ubiquitin linkage, the ubiquitin specific protease USP8 regulates the trafficking, sorting and stability of many membrane receptors at different levels of the endocytic pathway.
The work of the
Genetics and Chemogenomics
team of the Large Scale Biology Laboratory, conducted in collaboration with the IBS, showed that an endocytic protein (CHMP1B) is dynamically ubiquitinated within 5 to 10 minutes after cells stimulation with EGF, a cell growth factor (Figure). This ubiquitination is necessary for CHMP1B function in intracellular trafficking in human cells, as well as for wing morphogenesis in Drosophila flies. The results obtained by this team also show that CHMP1B is a target of USP8 which, by deubiquitating CHMP1B, may promote its polymerization at the endosome membrane in an endocytic complex known to trigger membrane deformation and scission (Figure).
Similar ubiquitin-dependent regulatory mechanisms may exist in all other processes that require deformation of the cell membrane, such as cytokinesis (cell division), viral budding, or autophagy. This is to say the importance of identifying therapeutic targets that control the physiological or pathological activity of proteins acting in the endocytic pathway.
The
Genetics and Chemogenomics team is interested in continuing the study of USP8 function and to select chemical inhibitors in a context where constitutively active mutant forms of USP8 are expressed in patients with Cushing's disease and overexpressed USP8 contributes to chemoresistance in cancer cells.
* Ubiquitin is a small polypeptide of 72 amino acids that is highly conserved during evolution, ubiquitously expressed in cells and capable of binding to target proteins
via a covalent bond on the free NH2 moiety of a lysine residue. Since ubiquitin contains lysine residues, it can polymerize into different types of polyubiquitin chains. This post-translational modification of proteins acts on their activity or stability and can also promote the formation of protein complexes.
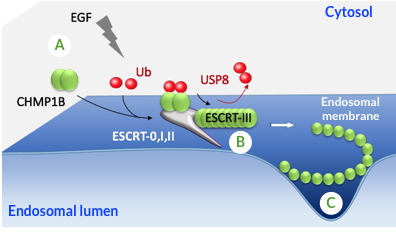
A - The CHMP1B protein is ubiquitinated and recruited at the endosome membrane after stimulation with EGF.
B - The hydrolysis of ubiquitin by USP8 would allow the polymerization of CHMP1B and
C - the deformation of the membrane of endosomes.