Biocatalysis is the biology of sustainable chemical synthesis, where the chiral catalyst can be an enzyme extracted from a living organism or a synthetic enzyme. For example, artificial metalloenzymes are developed by introducing a bio-inspired inorganic complex into a protein, in a cavity. The inorganic complex is the reactive center and the protein is the support for stability and selectivity. The aim is to develop efficient hybrid catalysts for oxidation reactions with a focus on asymmetric oxygen transfers.
Researchers at IRIG, in collaboration with the Department of Molecular Chemistry – Unité Mixte de Recherche CNRS & Grenoble Alpes University, are studying hybrids formed from various metal complexes (Co, Fe, Mn, Ru) and the NikA protein, which transports nickel in E. coli bacteria. These hybrids catalyze oxidation reactions of alkenes (CnH2n) or thioethers (R-S-R'). However, these reactions are only weakly stereoselective. In order to improve the enantioselectivity of the sulfoxidation reaction, i.e. the selection of the R and S forms of both enantiomers, the researchers replaced the NikA protein with a G-quadruplex oligonucleotide. Thanks to significant folding polymorphism, these G-quadruplexes, associated with a copper complex, constitute versatile DNA-based catalytic entities for selective asymmetric oxidation reactions. The enantioselectivity of the catalysis depends on the topology adopted by the G-quadruplex, and therefore on the reaction conditions. The researchers have shown that it is possible to constrain a G-quadruplex in a unique topology by attaching it to a polypeptide leading to the oxidation of thioanisole derivatives with an enantiomeric excess of up to 73% in the presence of hydrogen peroxide as the oxidant. Comparative studies between the natural G-quadruplex and constrained G-quadruplexes modified at the 3' external tetrad by the addition of one to six thymidines identified the different reaction sites within the artificial enzyme and proposed a reaction mechanism.
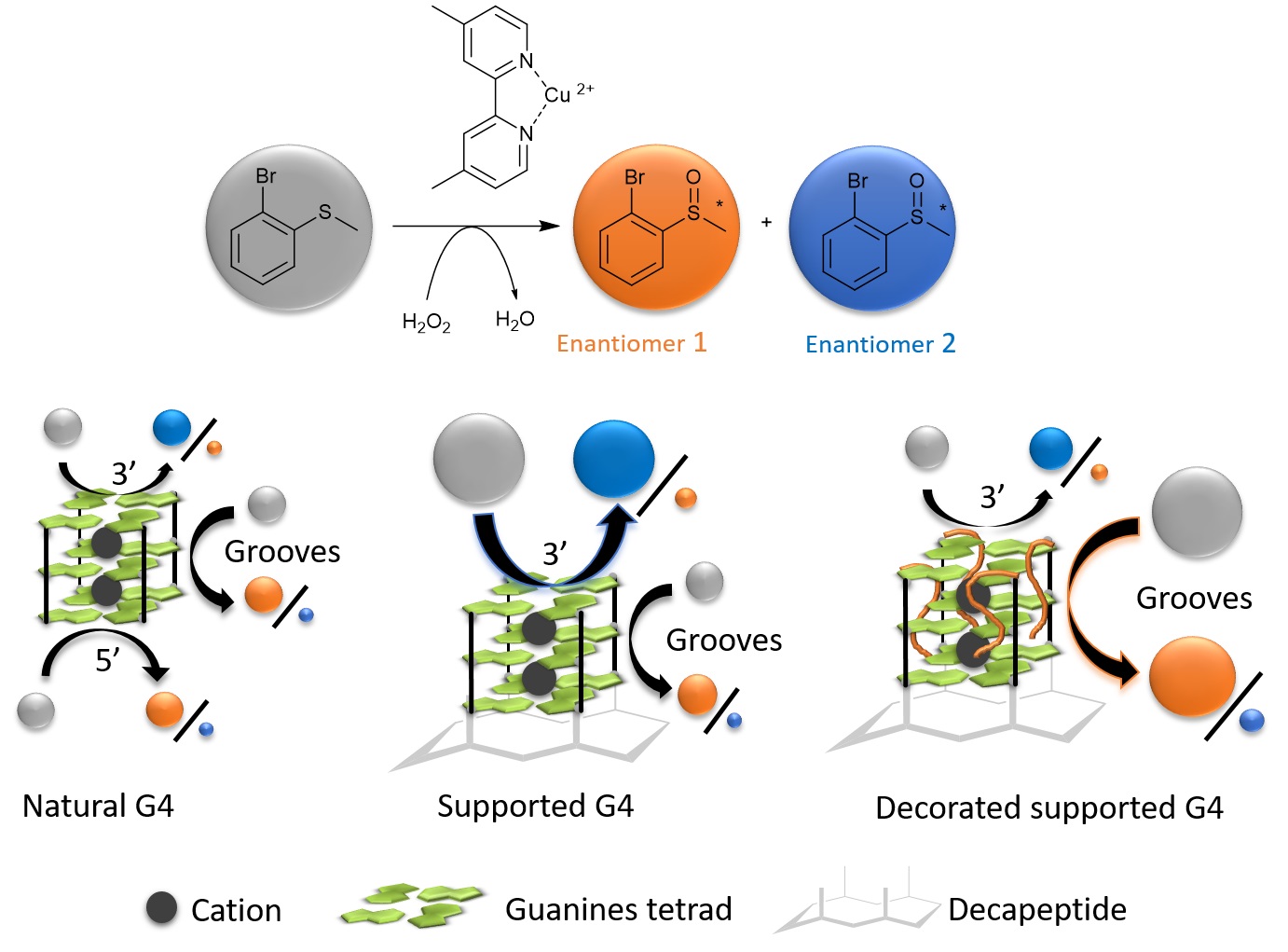
Figure: comparison of the enantioselectivity of an asymmetric sulfoxidation reaction as a function of the nature of the G-quadruplex catalyst.
The results obtained help to decipher the enantioselective control of the sulfoxidation reaction with G-quadruplex type catalysts, highlighting the importance of the nature of additional nucleosides over the 3’-tetrad.
Fundings: ANR CoolCat project.
Sulfoxidation adds an oxygen atom to a sulfur atom by a chemical process transforming a thioether into a sulfoxide and creating an asymmetric center on the molecule.
Two enantiomers R and S have the same physicochemical properties in relation to an achiral agent, but behave differently in relation to a chiral agent. The notion of chirality is essential in the molecular recognition processes of living organisms, for example in the functioning of enzymes or the action of drugs. It is therefore important for a chemist to preferentially synthesize a single enantiomer of an asymmetric molecule.