Phytoplankton play an essential role in sustaining life on Earth. By converting CO
2, sunlight and nutrients into biomass and oxygen, the single-celled organisms that make up phytoplankton are responsible for about 50% of primary production. They contribute to food chains and to the biological pump, a major component of the marine carbon cycle that allows the fixation of CO
2 in the oceans by sedimentation of microalgae. Understanding the cellular basis of phytoplankton response to environmental changes could inspire promising developments in biotechnology.
To date, the morphological characteristics of phytoplankton have been visualized primarily by light microscopy, three-dimensional (3D) confocal microscopy, and two-dimensional (2D) electron microscopy studies. However, these studies provide images with insufficient resolution to reveal cellular ultrastructure, and 2D electron microscopy cannot provide a complete volumetric description of these cells and their organelles.
A collaboration between several IRIG laboratories (LPCV, IBS, MEM) allowed the 3D reconstruction of the cellular architecture of eukaryotic cells representative of the main phytoplankton families (
Figure), using focused ion beam scanning electron microscopy (FIB-SEM)
[1]. The FIB-SEM images constitute a unique resource to identify the properties of subcellular architectures that have been maintained during the evolution of plankton. These include, the conservation of the volume occupied by the main organelles, and the preservation of the volumetric relationships and proximity between the energy-producing organelles mitochondria and plastids.
By revealing how an intimate interaction between cellular structures and physiological responses allows cells to adapt to various environmental conditions, these results shed light on the cellular basis of the flexibility of phytoplankton energy metabolism. Thanks to the combination of many expertises, researchers have shown the existence of a close link between the mitochondria-plastid interactions and the capacity to produce of algal biomass in mixotrophy (
i.e. the synergy between respiration and photosynthesis) in the extremophilic microalga
Galdieria sulphuraria [2], the model diatom
Phaeodactylum tricornutum [3] and the oleaginous microalga
Microchloropsis gaditana [1, 4] were characterized.
This study shows that the physiological responses of phytoplankton are based on specific characteristics of their main organelles (plastids and mitochondria), opening up perspectives of exploitation in the field of algal biomass production upstream of biotechnology applications.
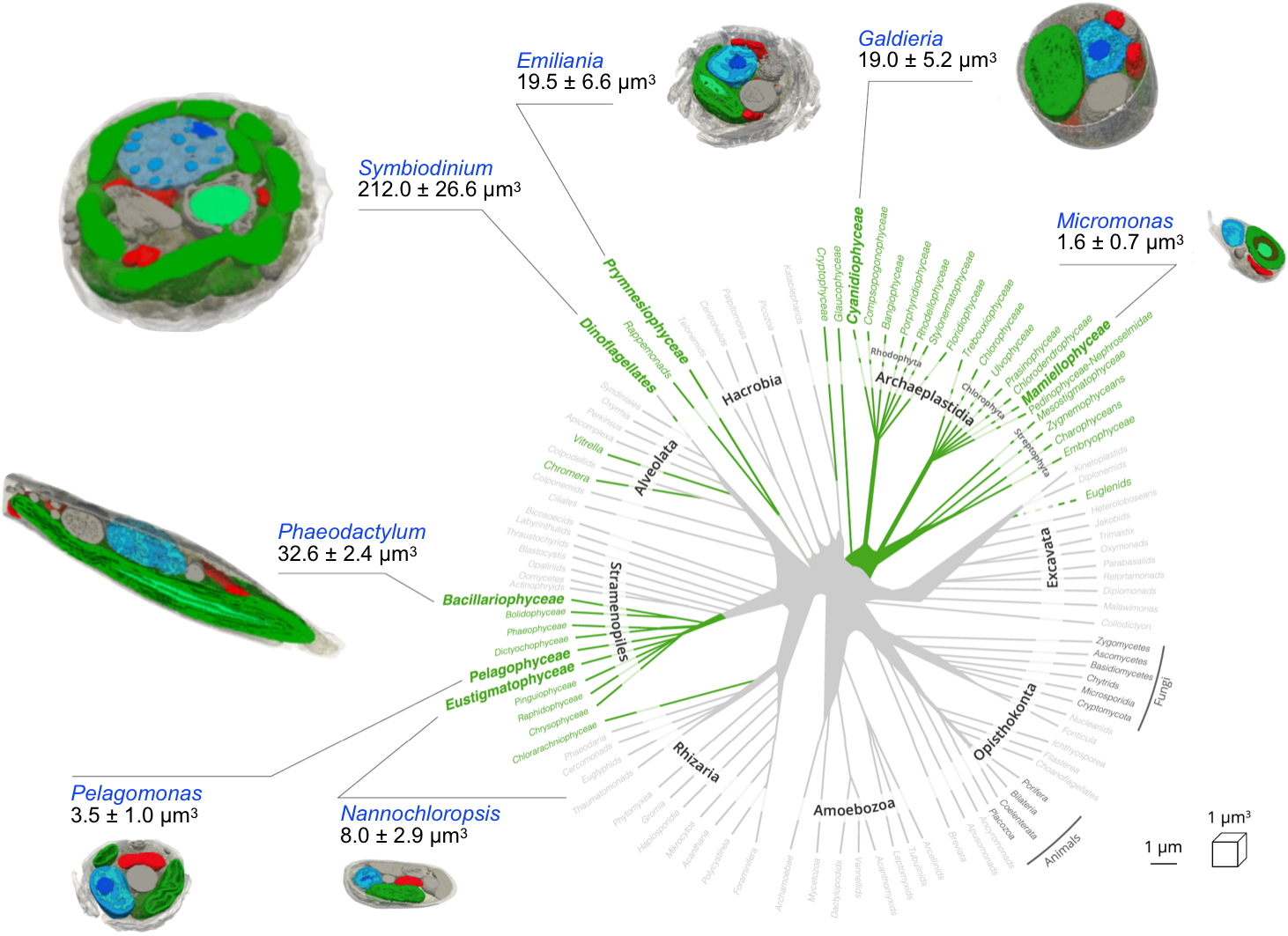
Internal cellular architecture of phytoplankton cells obtained from whole-cell FIB-SEM images.
The structures reveal the main subcellular compartments: plastids (green), mitochondria (red), nucleus (blue), other compartments (grey), and allow to calculate their volume.
Primary production is the rate at which energy is converted into organic substances by terrestrial and aquatic photosynthetic (photoautotrophic) organisms.
These expertises involved cryo-electron tomography performed at the MEM laboratory and at the IBS, photophysiology, lipidomics and metabolic engineering performed at the LPCV, proteomics at the Biosanté laboratory, metabolomics at the Heinrich Heine University of Düsseldorf in Germany and at the University of Liège in Belgium, and finally metabolic modelling at Oxford Brookes University in the United Kingdom.
These studies were carried out thanks to the ANR "MoMix" funding, the European Community (ERC AdG "Chloromito") and through collaborations with the Fermentalg [3] company and the Total petrochemical group [4].
Contacts:
Giovanni Finazzi, LPCV
Imaging: Denis Falconet, Johan Decelle, Pierre-Henri Jouneau, Guy Schoehn
Energetic metabolism: Gilles Curien