Lithium-ion batteries are increasingly common in our everyday lives. Their high-energy density, made possible by the lightweight element lithium, has rendered them essential to portable electronics. In the future, they may be used in electronic vehicles that will be powered “by sun” or “by wind.”
With this in mind, it is interesting to see what happens when batteries are combined with supercapacitors—storage devices capable of rapidly delivering a large amount of energy. More durable than the battery alone, the pair lends itself well to new functionalities such as stop-start technology and “dynamic” braking, in which the motor produces energy rather than consuming it.
Improving Li-Ion batteries autonomy and security
How do lithium-ion batteries and supercapacitors work?
These two electronic storage devices both contain twoelectrodes soaked in an electrolyte containing ions and separated by a membrane that is impermeable to electrons and permeable to ions.
- A lithium-ion battery is an electrochemical accumulator based on reversible lithium-ion exchanges between the positive electrode (cathode), most often composed of a cobalt oxide, manganese and nickel, and a negative electrode (anode), usually made of graphite. During discharge, the graphite releases Li+ ions that bind to the cobalt oxide with which they have a strong chemical attraction. The opposite happens during charging.
- In a supercapacitor, an electric double layer with opposing polarity develops during charging at the interface between each electrode and the electrolyte, and disappears during discharge.
Lithium-ion batteries in their current form must improve in two areas: autonomy and security. In particular, their charge capacity could, in theory, multiply tenfold if silicon were substituted for the negative electrode material (graphite). Rather than using bulk silicon, which swells excessively during charging, leading to major electrode damage, researchers plan to blend low-cost silicon nanoparticles with graphite.
The goal of the BACCARA project (Battery and supercapacitor characterization and testing) was to better understand the mechanisms that limit the performance of this type of battery, as well as that of a supercapacitor in which graphite is replaced by monoatomic-thick layers of carbon (graphene).
Multi-scale analyses of electrolyte and electrode interfaces
 “We have developed multi-scale analytical methods to observe the electrolyte/electrode interfaces, as well as their evolution over the course of charge/discharge cycles,”
“We have developed multi-scale analytical methods to observe the electrolyte/electrode interfaces, as well as their evolution over the course of charge/discharge cycles,” explains Pascale Bayle-Guillemaud, BACCARA coordinator and researcher at CEA-
INAC .
“Indeed, charge capacity, as well as battery and supercapacitor lifespan, play out at this level. This is even truer when using nanostructured materials in which the surface exposed to the electrolyte is much greater.”
Indeed, in the case of batteries, a solid residue called SEI (Solid Electrolyte Interphase) forms on the silicon, resulting from chemical reduction of the electrolyte. To study this undesirable process, researchers at CEA-INAC developed advanced structural, morphological and spectroscopic analyses of functional devices. Mock-ups cells of batteries and supercapacitors were specially developed for operando observation by electron microscopy, X-ray reflectivity and diffraction performed at the european synchrotron ESRF.

"Nanomil" platform for the synthesis of nanostructured materials for Li-ion batteries dedicated to industrial transfer.
Handling (weighing and filling of a crushing bowl) of nanometric or sensitive powders in glovebox under controlled atmosphere in depression. © P.Avavian/CEA
Slowing better-understood decay
The scientists concluded that the SEI layer forms during the battery’s early cycles, while the organic solvents in the electrolyte continuously deteriorate until all the Li+ ions are trapped in the SEI layer or in the altered electrolyte.
The researchers at BACCARA were able to highlight the mechanisms that decrease device performance over the course of cycling and suggest electrochemical models, which have been published in the articles referenced below. They were also able to test several electrolytes, laboratory devices and industrial prototypes, as well as solutions that improve cell cycling and safety.
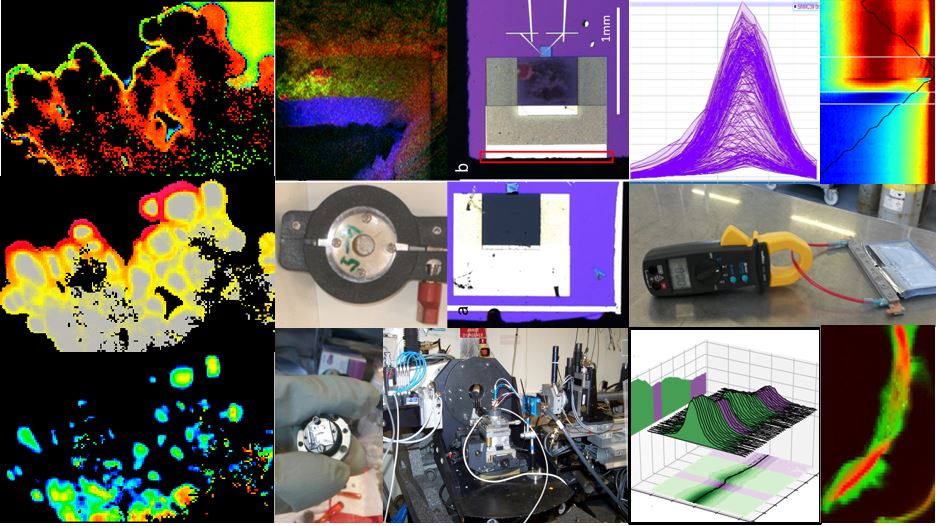
Multi-scale and multi-techniques characterization of Baccara materials and cells. © CEA & Hutchinson
BACCARA—to which CEA-
LITEN contributed an analytical method—brought to light CEA-INAC's expertise regarding advanced battery characterization. Unsurprisingly, CEA-INAC waschosen as a partner by Varta Micro Innovation, an Austrian industry player involved with BACCARA, for
another European H2020 project, Sintbat, with more industry-oriented objectives than BACCARA. In Sintbat, CEA-INAC is of course overseeing the work related to battery decay.
BACCARA

Coordinated by the CEA from October 2013 to 2016, this project involved six partners from 5 countries. The total budget was of 3.8 million euros, dedicated to improve the performance of LiB and supercapacitors.