The researchers decrypted the operating mechanisms of FAP (Fatty Acid Photodecarboxylase), which is naturally present in microscopic algae such as Chlorella. The enzyme had been identified in 2017 as able to use light energy to form hydrocarbons from fatty acids produced by these microalgae. To achieve this new result, research teams used a complete experimental and theoretical toolkit.
Understanding how FAP works is essential because this photoenzyme opens up a new opportunity for sustainable biofuel production from fatty acids naturally produced by living organisms. FAP is also very promising for producing high added-value compounds for fine chemistry, cosmetics and pharmaceutics.
In addition, due to their light-induced reaction, photoenzymes give access to ultrarapid phenomena that occur during enzymatic reactions. FAP therefore offers a unique opportunity to understand in detail a chemical reaction taking place in living organisms.
More specifically, in this work, researchers show that when FAP is illuminated and absorbs a photon, an electron is stripped in 300 picoseconds from the fatty acid produced by the algae. This fatty acid is then dissociated into a hydrocarbon precursor and carbon dioxide (CO2). Most of the CO2 generated is then turned in 100 nanoseconds into bicarbonate (HCO3-) within the enzyme. This activity uses light but does not prevent photosynthesis: the flavin molecule within the FAP, which absorbs the photon, is bent. This conformation shifts the molecule's absorption spectrum towards the red, so that it uses photons not used for the microalgae's photosynthetic activity.
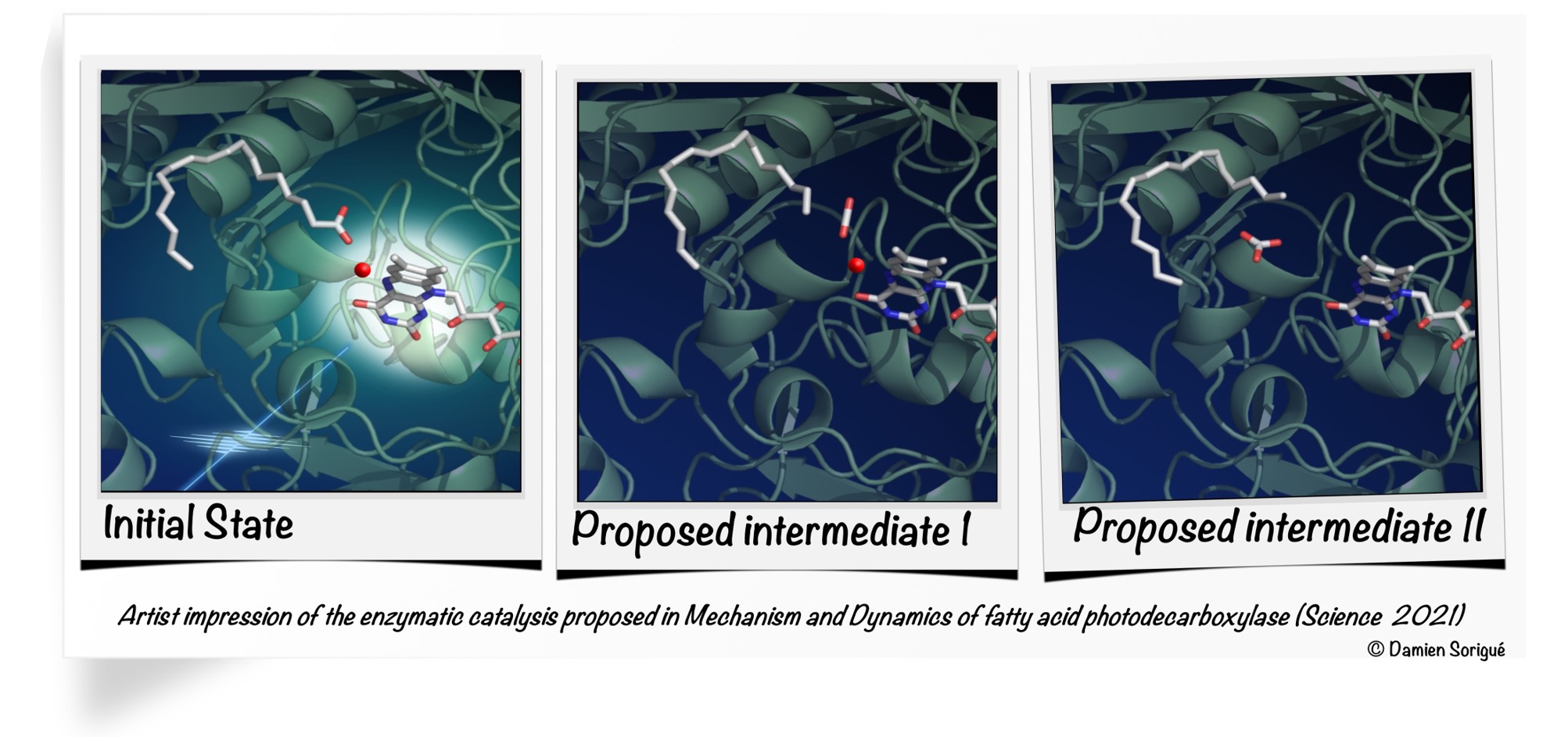
It is the combined interpretation of the results of various experimental and theoretical approaches by the international consortium that yields the detailed, atomic-scale picture of FAP at work. This multidisciplinary study combined bioengineering work, optical and vibrational spectroscopy, static and kinetic crystallography performed with synchrotrons or an X-ray free electron laser, as well as quantum chemistry calculations.
In France[1], the study involved researchers from the Biosciences and Biotechnologies Institute of Aix-Marseille (CEA/CNRS/Aix-Marseille University), the Institute of Structural Biology (CEA/CNRS/Grenoble Alpes University), the Laboratory for Optics and Biosciences (CNRS/École Polytechnique-Institut Polytechnique de Paris/Inserm), the Advanced Spectroscopy Laboratory for Interactions, Reactivity and the Environment (CNRS/University of Lille), the Institute for Integrative Biology of the Cell (CEA/CNRS/Paris-Saclay University), the SOLEIL synchrotron and also from the European Synchrotron (ESRF) and the Laue Langevin Institute (ILL), two major European instruments based in Grenoble. It received funding from the French National Research Agency.
[1] The study also involved researchers from the Max Planck Institute in Heidelberg (Germany), Moscow State University (Russia) and the SLAC National Accelerator Laboratory (USA).