While vaccines target the entry of the virus into the cell via the spike protein, it would be interesting to also be able to target the replication machinery of the virus, so as to treat individuals already infected.
This is why CEA-Irig researchers (at the Institute of Structural Biology in Grenoble) have chosen to study the nucleoprotein N of SARS-CoV-2, one of the virus's most abundant proteins. This nucleoprotein is important to several vital functions:
- it protects the viral genome from the host's intracellular immune system
- it is a major component of the virus replication-transcription complex
This natural target for developing an antiviral treatment contains long, disordered regions that give it great flexibility – the key to its biochemical action – but also make it extremely challenging to study. For this reason, the protein remains poorly characterized outside of its structured domains.
The IBS researchers – experts in NMR (nuclear magnetic resonance) spectroscopy, which gives them access to atomic resolution even in the case of very dynamic systems – have succeeded in describing:
- the structure and dynamics of N in the presence of the disordered domains
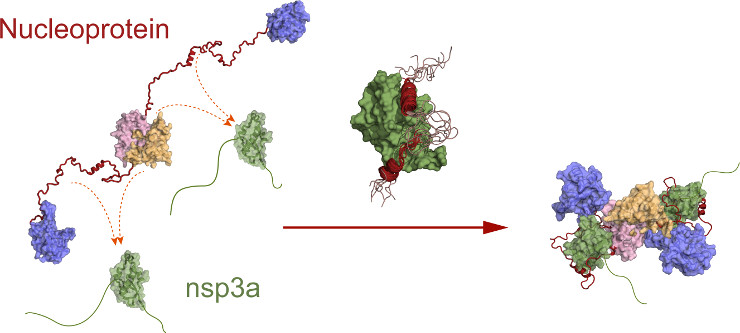
- its interaction with the viral protein nsp3a
This interaction, which places the N protein at the site of viral genome production, is crucial for virus replication. It involves two "linear motifs" of the central disordered domain of N, which envelop the nsp3a protein by folding the disordered domain around the partner. This results in a substantial reduction in the volume of N. The two proteins therefore form a very compact molecular assembly that can regulate the essential interaction of N with the viral RNA.
This work provides insight into the mechanism of virus replication and opens the way to the development of new strategies against COVID-19, for example by inhibiting this important interaction for viral replication.