Despite their life expectancy of 1 to 3 years, tardigrades are the only animals known to be able to withstand the vacuum of space and the lack of oxygen. Their remarkable ability to survive extreme conditions of stress (desiccation, temperature, radiations, etc.) has attracted considerable interest in the origin of this resistance. Recently, it has been shown that these animals express intrinsically disordered proteins that play an essential role in anhydrobiosis, a physiological state that allows them to survive in a dormant state for decades. These proteins are remarkable in that they lack a persistent three-dimensional structure, yet still function in their disordered state..
To better understand how intrinsically disordered proteins specific to tardigrades might confer protective properties in response to desiccation and other environmental stresses, researchers at IRIG, have just characterized the conformational and physical behavior of
one of these proteins, the CAHS-8 protein, using primarily Nuclear Magnetic Resonance (NMR) and Atomic Force Microscopy (AFM), combined with other biophysical techniques.
They were able to determine at the atomic scale that the CAHS-8 protein has disordered and highly flexible arms surrounding a long central helical domain whose behavior is highly dependent on temperature. Based on their results and observations, the researchers propose that these proteins associate
via part of this helical domain in response to environmental changes (low temperature, high concentration). This reversible association, which could be imaged, leads to the formation of oligomers, then fibrils. These fibrils end up forming a gel with pores of regular size. In addition to studying the gel itself, the IRIG researchers were also able to sequester other proteins in the gel and to study their behaviour. Although trapped in this CAHS-8 matrix, the structure of these proteins is not modified. However, their movements are significantly slowed down. The exact mechanism by which the formation of the gel protects the tardigrade remains unknown, but it is possible that the formation of such an intracellular matrix allows the maintenance of the biomolecules in their functional state by reducing, for example, the volume of water that they require. Cryoprotection would also be facilitated by this means, reducing by the same mechanism the possibility of ice crystal formation.
In this study, IRIG researchers present a detailed characterization of the transformation of the physical properties of the CAHS-8 protein in response to environmental conditions. They propose that the observed transformation of this important protein from a soluble monomer to the formation of a matrix may be responsible for a protective role in the dramatic response of tardigrades to environmental stress.
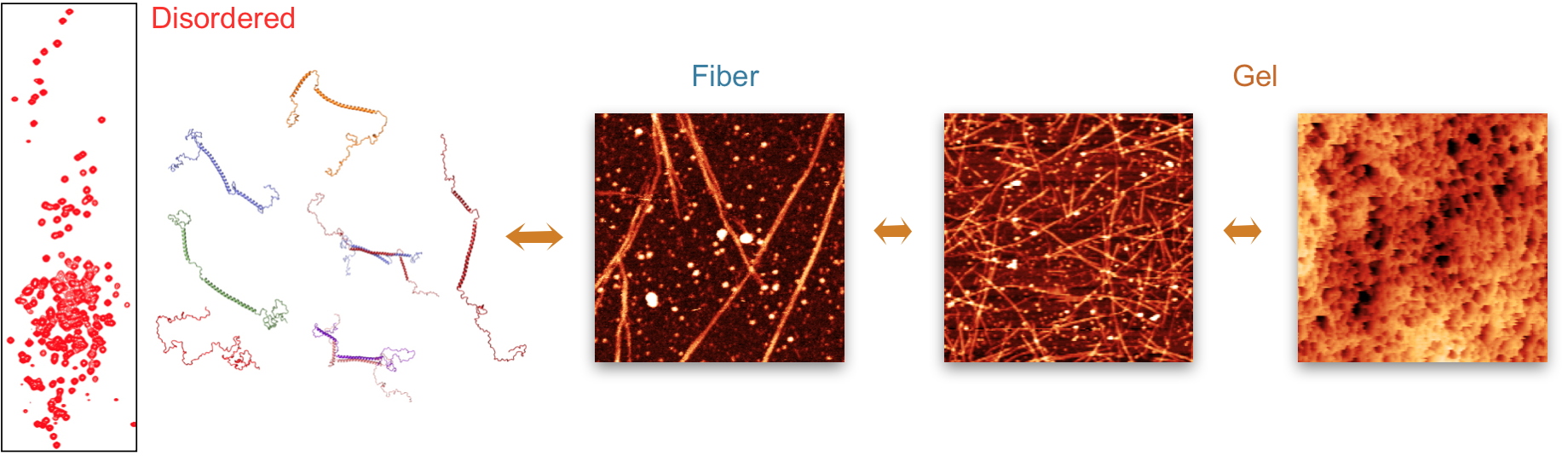
NMR spectroscopy reveals that the protein comprises a central helical domain, flanked by disordered termini. After concentration of the protein, it forms successively oligomers, long fibers and finally gels consisting of fibers, all in a strongly temperature dependent manner. Credit CEA
A Cytosolic Abundant Heat-Soluble protein, CAHS-8 from Hypsibius exemplaris which is a tardigrade species.