Transition metals play a key role in protein function. Thanks to their unique properties enzymes can afford reactions otherwise impossible. Hence, the so-called SAM Radical proteins form a superfamily of metalloenzymes that use a [4Fe-4S]
+ active center to cleave S-adenosyl-L-methionine (SAM) and generate a 5´-deoxyadenosyl radical. This high-energy radical enriches the panoply of possible chemical reactions, leading to the use of "SAM Radical" enzymes for cofactor biosynthesis, peptide modification, or even antibiotic synthesis.
Because of their potential, metalloenzymes can be considered as biotechnological tools. Thus, some research aims at designing new artificial enzymes. This research focuses notably on understanding how the protein backbone controls the the high-energy intermediates that are involved in catalysis.
The work carried out by the IRIG researchers is part of this context. These researchers studied ThiH and NosL, two SAM radical proteins. These enzymes have the same primary structure as well as similar substrate binding modes. Therefore, comparing how they break alternative C-C bonds should lead to a better understanding of the mechanisms involved. The crystal structure of ThiH highlighted an unusual protonation state of its substrate L-tyrosine. Moreover, the quantum modeling of the reactions has revealed a
tunneling effect lowering the activation barrier of the reaction. Finally, subtle structural changes between the two enzymes affect this activation, leading to the difference in specificity of the reaction and thus to different C-C bond breaks.
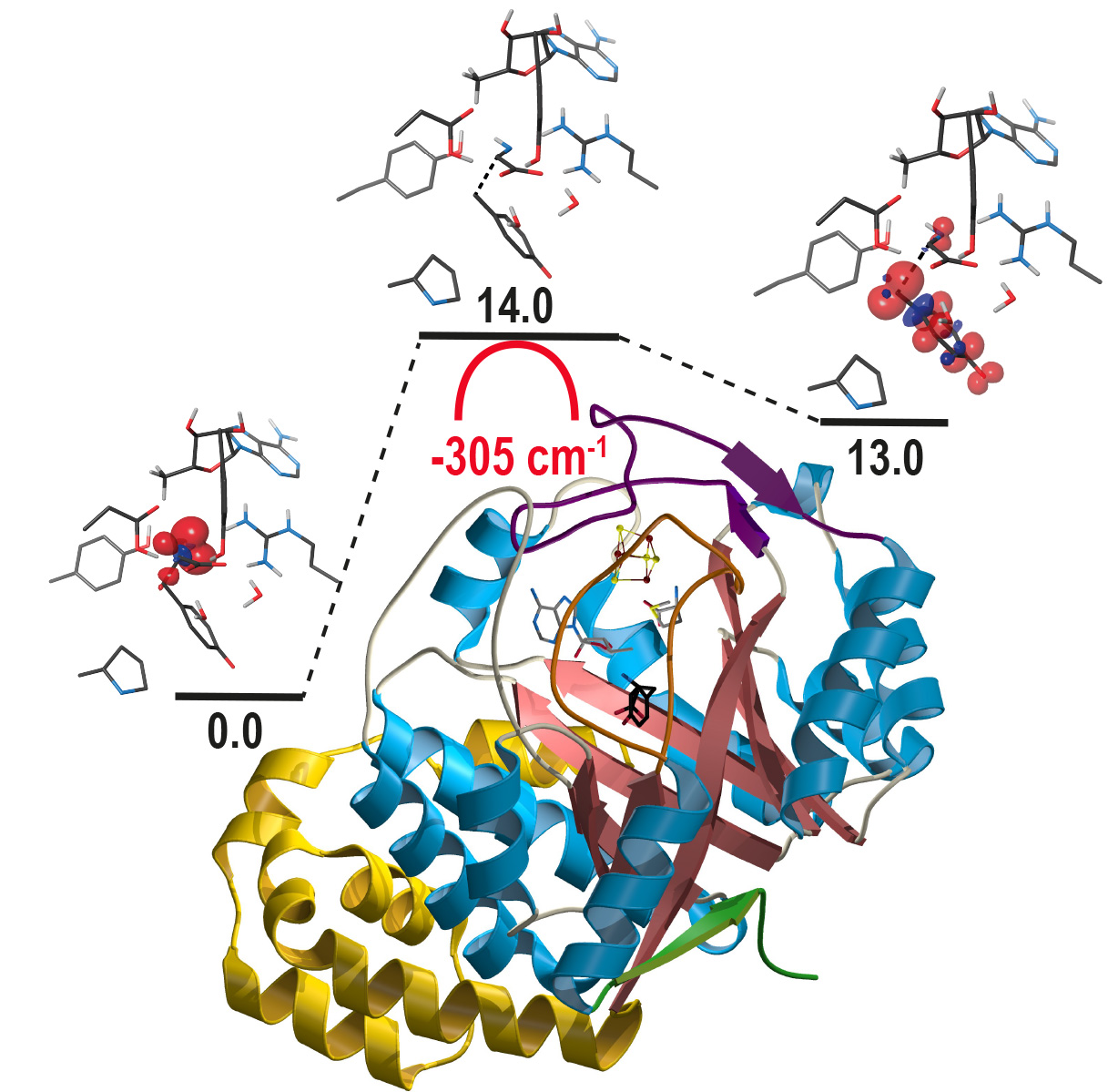
Structure of the ThiH protein under the energy profile of the reaction. Credit CEA
Tunneling effect: A quantum phenomenon, which cannot be explained by classical mechanics. It allows a quantum object to pass a potential barrier even if its energy is lower than the minimum energy required to overcome this barrier.