Placental cancer, or gestational choriocarcinoma, can unfortunately cause maternal death even several years after the pregnancy. Researchers at IRIG are studying the behavior of the NLRP7 protein involved in inflammatory processes suspected in the development of this cancer. In 2021, they had shown that the overexpression of NLRP7 in placental cancer cells contributed to their metastasis to other organs, such as the liver, the lung or the brain.
Continuing this work, researchers at IRIG first compared the mechanisms of NLRP7 protein function in a normal placenta cell and in a cancer placenta cell. The normal cell was collected from the placenta of a pregnant woman, and the tumor cell was recovered from a patient who had died of choriocarcinoma.
In the normal cell (Figure A), the NLRP7 protein functions in a mode dependent upon its inflammatory machinery called, inflammasome, which allows the production of pro-inflammatory cytokines, such as interleukin-1β (IL-1β).
In the tumor cell (Figure B), the researchers showed that overexpression of NLRP7 blocks the activation of the NF-κB protein, that allows to produce IL-1β precursor, the pro-IL-1β, wich drives NLRP7 to function in an inflammasome independant pathway.
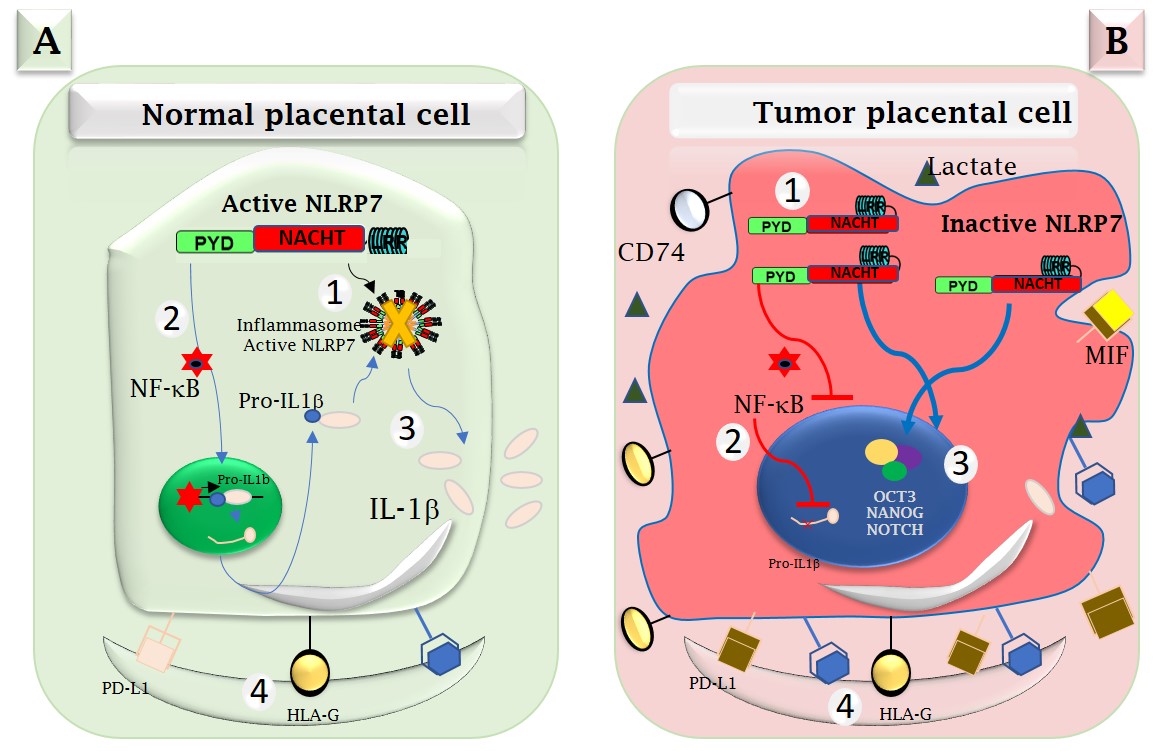
A summary model illustrating the mechanism by which NLRP7 contributes to choriocarcinoma tumorigenesis. Credit CEA
Figure A: In non-tumor trophoblast cells, NLRP7 is expressed at normal levels and functions in an inflammasome-dependent manner. In these cells, NLRP7 activates the NF-κB pathway and induces its translocation to the nucleus, which in turn induces the transcription of Pro-IL-1β that matures into IL-1β. NLRP7 also regulates the expression of HLA-G and PD-L1, contributing to trophoblast tolerance by the maternal immune system. All these events contribute to the safe progression of the pregnancy.
Figure B: In tumor trophoblast cells, NLRP7 is overexpressed and functions in an inflammasome-independent manner. It inhibits IL-1β production by decreasing NF-kB activation. NLRP7 also mediates the overexpression of HLA-G, PD-L1 and OCT3, NANOG and NOTCH proteins. These events increase the maternal immune tolerance of tumor cells, which creates a favorable anti-inflammatory environment that contributes to tumor growth.
In addition, the researchers demonstrated that NLRP7 increases tumor cell survival and camouflage. Finally, the researchers injected choriocarcinoma tumor cells expressing or not the Nlrp7 gene into the tail of a mouse. The results showed that cells overexpressing Nlrp7 were less exposed to the mouse's immune defense system, and that mice injected with these cells developed larger tumors and had more metastases. Analysis of the tumors and their environments confirmed the decrease in the activation of the host immune system.
These studies demonstrated that NLRP7 contributes to the growth and tumorigenesis of placental cancer. NLRP7 contributes to the development of a mechanism that impedes tumor clearance by the maternal immune system.
These findings suggest that the targeting of NLRP7 may pave the way for new therapies to be proposed to patients with choriocarcinoma, in particular, those who develop resistance to conventional treatments.