Ten years ago, it came as a surprise that vitamin B12 derivatives have been repurposed for light detection by a large family of previously unknown photoreceptors in bacteria, which perform various functions.
A prototype vitamin B12 photoreceptor, CarH, regulates the expression of genes involved in protecting bacteria from excessive sunlight. It does so by binding to DNA in the dark, acting as a molecular stopper. When exposed to light, its tetrameric architecture breaks down, enabling transcription by detaching from the DNA.
However, the precise molecular functioning of this photoreceptor and other vitamin B12 photoreceptors has remained a mystery ever since. An international consortium led by scientists from CEA-Irig/IBS has now combined experimental techniques using X-ray free-electron lasers (XFEL) in Switzerland (SwissFEL) and Japan (SACLA), as well as the synchrotrons in Grenoble (ESRF) and England (DLS), with quantum chemistry calculations to elucidate the molecular functioning of CarH.
After triggering photoactivity in CarH using an intense pulse of visible laser light, the researchers observed structural changes in the photoreceptor in real time. From the first moments after light absorption on the nanosecond scale to the loss of the photoreceptor's tetrameric architecture, the study reveals the sequence of orchestrated molecular events underlying its functioning. Surprisingly, the researchers discovered an unexpected intermediate state that the photoreceptor transiently adopts during the reaction process. This state appears to protect the photoreceptor from returning to its initial state and directs it towards continuing the reaction. Such an intermediate state has not been observed in vitamin B12 enzymes, making it a plausible explanation for the light-detecting ability of vitamin B12 photoreceptors.
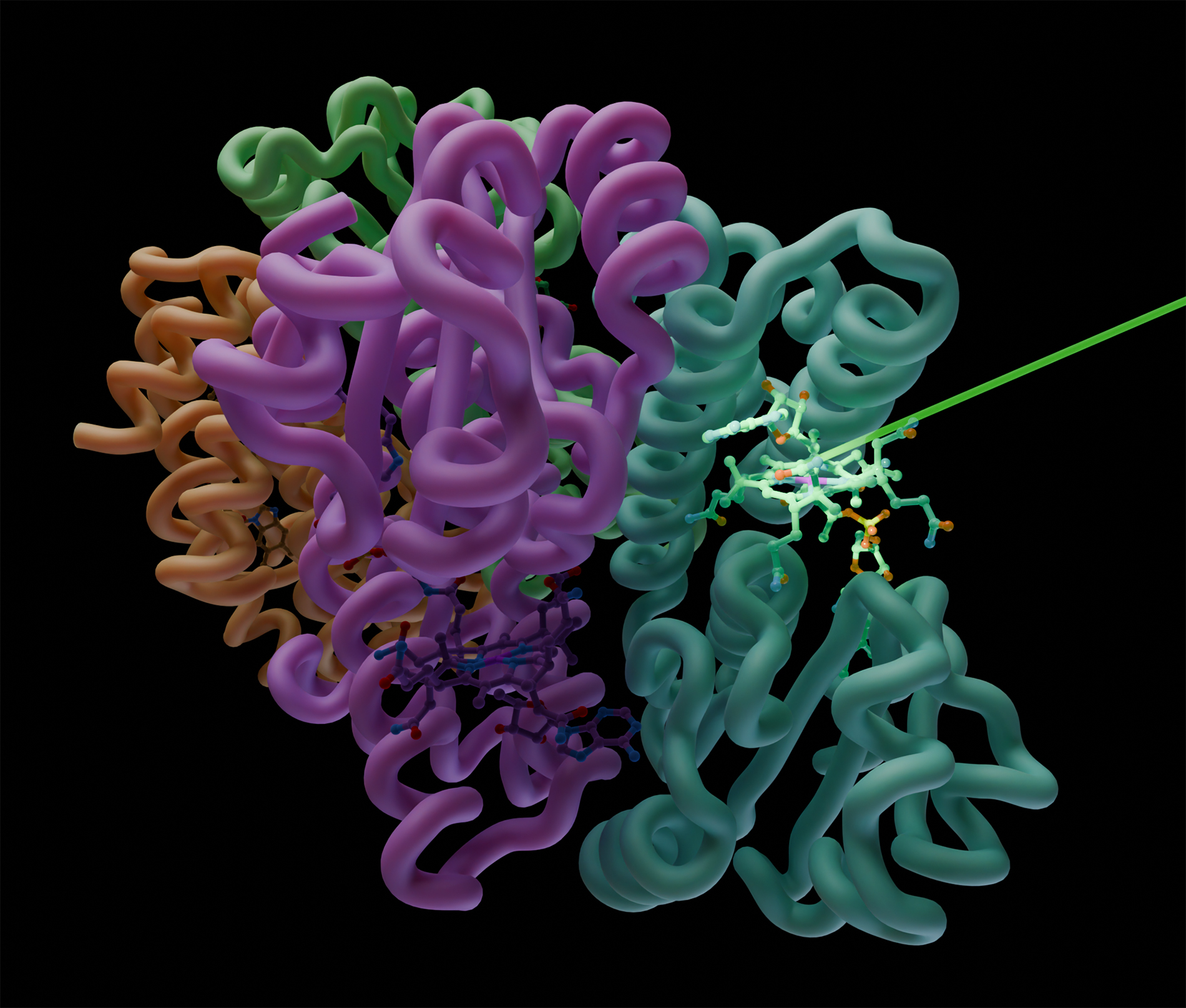
Figure : An
intense laser pulse photoexcites a vitamin B12 molecule within the CarH
photoreceptor. The resulting conformational changes lead to the disintegration
of its tetrameric architecture. © CEA-Irig/IBS/M. Davila
Understanding how CarH works at the molecular level will enable this photoreceptor to be modified for biotechnological applications, such as optogenetics, which involves controlling cellular processes using light.
organometallic cofactor*: organic molecule containing one or more metal ions, necessary for an enzyme to catalyse a given reaction.
photoreceptors*: light-sensitive proteins.
The IBS is a joint Research Unit (UMR 5075, CEA-CNRS-UGA).
The study was funded by the ANR, the CNRS, the Hubert Curien Partnership (PHC) Sakura, and the EPSRC, and involved PhD students funded by the CEA, GRAL, and ENS Paris.
The study was conducted as part of a collaboration between researchers from IBS, ESRF, the Universities of Manchester (England), Louisville (United States), Hyogo (Japan), Saarland (Germany), the XFELs at the Paul Scherrer Institute (Switzerland) and SACLA (Japan), and the DLS synchrotron (England).