Genome instability and the acquisition of genetic alterations that goes with it contribute to aging and tumor development. DNA lesion repair mechanisms are thus essential for cell and organism survival. Homologous recombination (HR) is one of these mechanisms, playing a central role in genome stability and repair. HR is very highly conserved; it is present in all living organisms, from bacteria to humans. The key protein in HR is the recombinase Rad51. On damaged DNA and with upstream help from such accessory proteins as BRCA2, It forms oligomers that enable the search for identical DNA sequences employable as matrices to ensure high-fidelity lesion repair.
Because of its role in genome stability, HR is traditionally considered to be a tumor suppression mechanism. Contributing to that mindset, numerous accessory genes in HR, particularly BRCA1 and BRCA2, are mutated in tumors. This is particularly the case in hereditary breast and ovarian cancers. Strangely, despite its key role in HR, inactivation of Rad51 is not observed in tumors, constituting what has been called the "Rad51 paradox."
One of the numerous hypotheses to explain this paradox is that mutated accessory proteins (such as BRCA1 and 2) prevent Rad51 from binding to DNA. This latter would thus be more accessible to other, nonconservative repair mechanisms that may prove more mutagenic. This hypothesis raises the following question: Which process drives tumor development: the deactivation of HR, or the ensuing stimulation of nonconservative repair mechanisms?
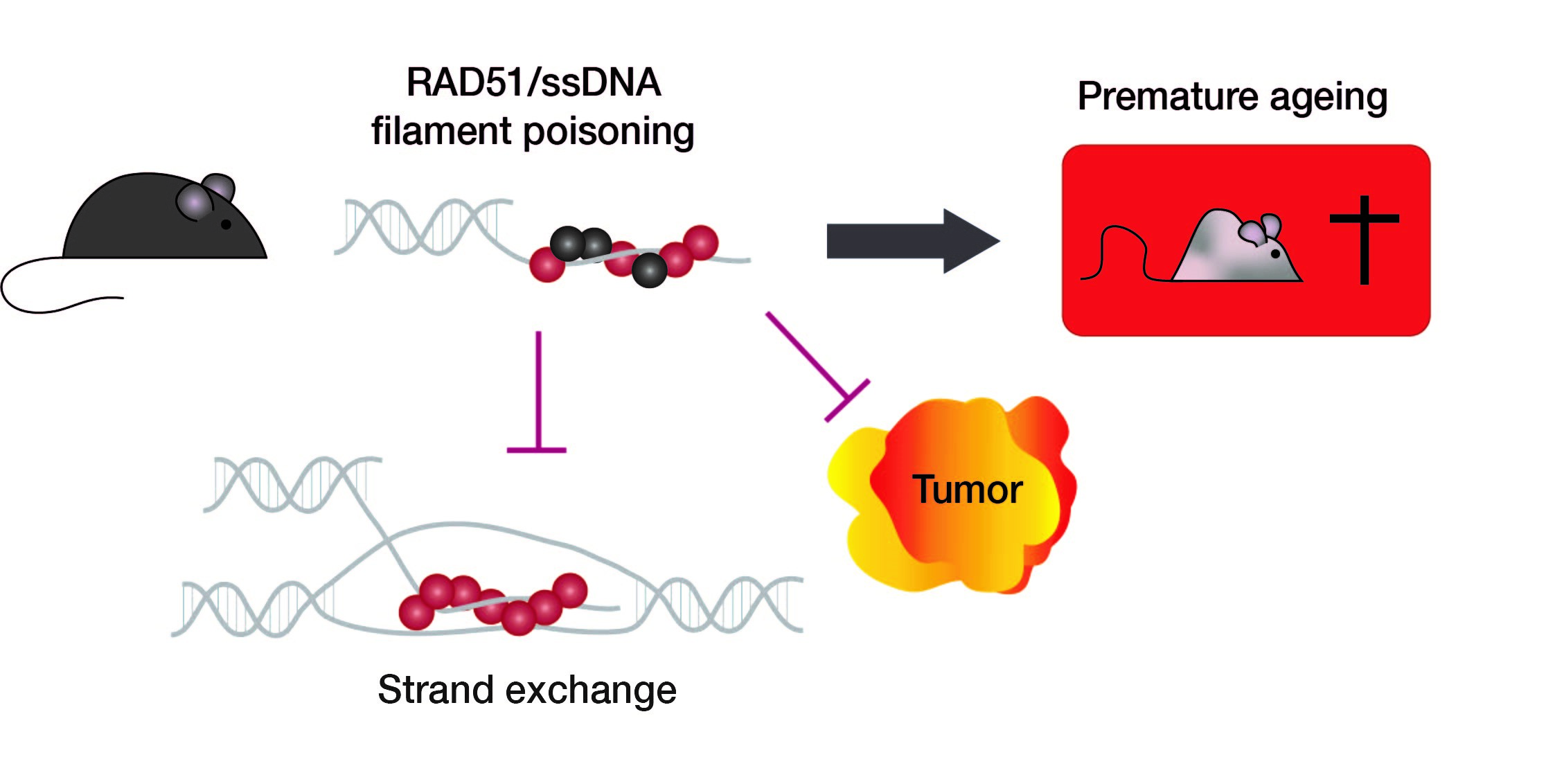
To answer that question, researchers from iRCM's Laboratory of Development of the Gonads partnered with Institut Cochin's Genome Stability and Instability team to develop a mouse model expressing a dominant-negative form of Rad51, called SMRad51. This latter is the product of an ectopically expressed chimeric gene. Once bound to the damaged DNA, SMRad51 inhibits not only endogenous Rad51-mediated HR, but also the other nonconservative, more mutagenic mechanisms of DNA repair. SMRad51 is thus a unique experimental tool enabling a genuine look at the results of Rad51-mediated HR deactivation, without the "interference" of those more mutagenic pathways.
Total inactivity of Rad51 leads to early embryonic death. Thus, the researchers built a murine model with conditional expression of SMRad51, i.e. a genetic construction wherein the chimeric gene only expresses in the presence of doxycycline. In this manner, they were able to activate or inactivate SMRad51 expression at any time in the mice's lives.
The team's results showed that HR suppression by SMRad51 caused replicative stress, systemic inflammation, progenitor cell exhaustion, early aging, and a reduced lifespan, but it did not cause tumor formation.
To better understand the role of SMRad51 in tumor development and in vivo, the researchers crossed their murine model with another murine model of breast cancer predisposition. Therein, the expression of SMRad51 diminished both the frequency and size of breast tumors.
In all, the study showed that preventing Rad51-mediated HR, while also making sure that the nonconservative, error-prone alternative mechanisms don't take its place, primarily results in aging effects rather than oncogenesis, and even more so, it seems to prevent tumor development.
© Bernard Lopez / CNRS / Institut Cochin
The work sheds new light on Rad51's important role in the balance between oncogenesis and aging, and suggests a possible competition, more so than cooperation, between these two processes.