The chemical industry relies on chemical catalysis to increase the yield and
selectivity of the reactions used in its production processes. The industry is
also looking into using enzymes, which are natural catalysts. However, these
biomolecules are fragile and can only be used for a limited number of reactions.
The idea of scientists is to take inspiration from their functional mechanisms,
to create artificial customized enzymes following the needs and constraints of
industry.
A team from the CEA-IRTSV in collaboration with the IBS (in Grenoble) has
mastered, for the first time, a method to construct an artificial metalloenzyme.
This enzyme is comprised of a backbone and an active site having an affinity for
a reagent, i.e. the molecule that will be transformed by the chemical
reaction. For a backbone, the researchers chose a protein without any activity
that is stable in relatively aggressive environments, and contains a pocket in
which the active site is located. This site, which is central to the chemical
reaction, contains a metallic iron-based molecule. The third and final player is
the reaction’s reagent, which must have an affinity with the enzyme. How can the
catalytic efficiency of this artificial enzyme be verified? The scientists have
sought out eligible reagents for a well-defined chemical reaction (that is also
used by the pharmaceutical industry), namely the transfer of an oxygen atom to a
sulfur atom. Starting from “molecular docking” calculations, 300 candidate
molecules were identified. Tests conducted on some of them show that the
reaction does occur at the active site of the artificial enzyme. In addition,
the number of catalytic cycles is much greater than that achievable by chemical
catalysis.
The findings represent a new stage in the development of artificial enzymes
derived from chemistry and biology, as well as for sustainable chemistry. The
first potential application: the development of anti-ulcer compounds, analogs of
drugs such as omeprazole.
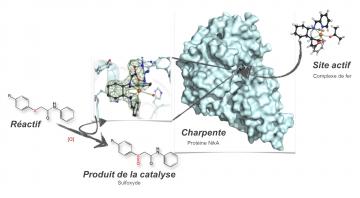
General
scheme for the synthesis and functioning of the metalloenzyme. The
product of catalysis is an analogue of omeprazole, a proton pump
inhibitor in the gastric mucosa of humans (used as an anti-ulcer drug).