Atmospheric
CO2 is the main greenhouse gas implicated in global warming. So why not
recover and give value to it, in particular by transforming it into a renewable source of energy? This would be a difficult operation due to its chemical stability, although certain microbial enzymes, e.g.
formate dehydrogenases (FDH),
catalyze its reduction into formic acid. The latter constitutes an interesting form of storage and energy transport: it can be broken down on demand to CO2 and hydrogen, for example to power a
fuel cell.
Before considering the technological applications of FDHs, it is essential to understand how they are synthesized and activated in the microbial cell. In particular, these enzymes include a
molybdenum cofactor that is only active in its sulfurous form. A team from the CEA-IBEB has just shown in
Escherichia coli how a chaperone protein1 provides the necessary sulfur atom. This step posed a small mystery since the cofactor is a very fragile compound and sulfur is a highly reactive element. By combining crystallization, X-ray diffraction, biochemistry and computer modeling, the researchers have proposed a novel molecular mechanism for this delicate transfer. The chaperone protein, in the form of a dimer, is bound on one side to the cofactor and on the other to a sulfur donor protein. Between the two, it forms a kind of tunnel through which passes sulfur. Strong sequence similarities suggest that a similar mechanism is at work in other bacteria.
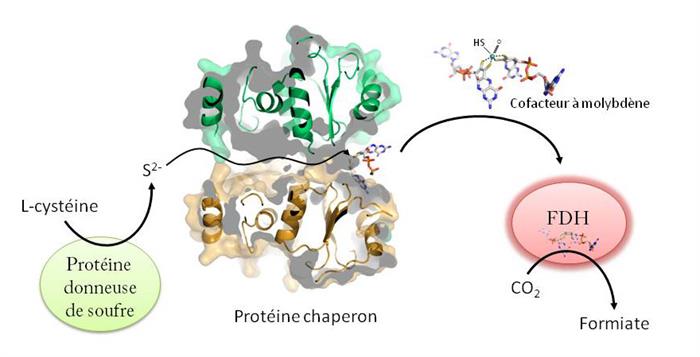
In this figure, the sulfur produced from L-cysteine navigates a tunnel through the chaperone protein to reach the molybdenum cofactor fixed on the other side of the protein. As soon as the molybdenum cofactor is sulfurous, it is available for FDHs and thereby enables their activity. © A. Magalon
[1] A protein involved in the maturation of other proteins.