In addition to their intrinsic genetic properties, cells within a tissue are subjected to a complex mixture of extrinsic physical stresses, signals and stimuli, emitted by their micro-environment. Although the cultivation of two dimensions (2D) human cells on plastic has led to major advances in biology, it very poorly models the reality of the cellular microenviron-ment in a tissue. As a consequence human biology is entering a new era where biologists are beginning to explore with enthusiasm the possibility of growing cells in flexible organic polymers, organizing them in 3D, to get closer to the physiological reality of the tissue.
Ex vivo models of 3D architecture (organoids, organs-on-a-chip) have the potential to fill the gap that exists today between 2D cellular culture and animal experimentation, which also presents limitations both at a bioethical level and as a model for human biology. Thus, prostatic or mammary epithelial organoids grown in Matrigel have strong similarities to the glandular tissue, as the development of acini perfectly polarized with a hollow lumen in their center. The possibility of genetically modifying these organoids would certainly bring new information on the organogenesis and carcinogenesis of these tissues.
As part of their work, researchers from the
Biomicrotechnology and Functional Genomics (Biomics)
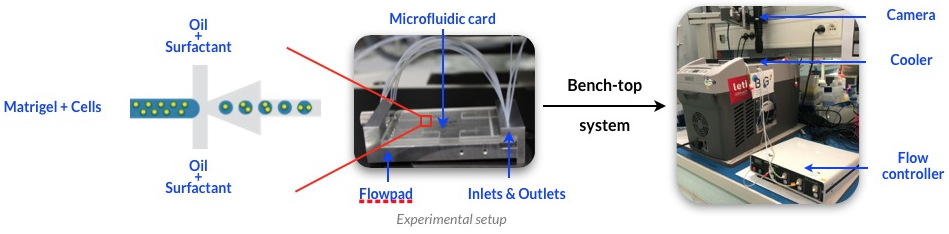
team of the Large Scale Biology Laboratory have developed an innovative approach to express transgenes in prostatic or mammary organoids by combining microfluidic encapsulation of single cells in Matrigel microbeads together with
electroporation of nucleic acids
(Figure).
Researchers have shown that electroporation of encapsulated organoids is nearly 80% effective. Using this approach and the morphological analysis of the generated organoids, they validated the key role of PTEN and p63 proteins in prostatic and mammary acetic development. They thus believe that the controlled generation of clonal organoids and their 3D transfection by nucleic acids, open new perspectives for the realization of functional genomic screens with high-throughput and high information content on human models that are physiologically more relevant than 2D cell culture.
Electroporation is a technique that makes it possible to force a cell to integrate foreign nucleic acid.