Metals are essential for cell function. In order to make them cross the cell membrane, specialized membrane proteins will either import them, in case of deficiency for example, or export them when they are at concentrations potentially toxic for the cell. Present in all living organisms, P1B-ATPases constitute one of the membrane proteins families specialized in the transport of metals, whether they are toxic, such as cadmium and lead, or essential at low concentrations and toxic at high concentrations, such as zinc and copper. The P
1B-ATPases share the same enzymatic mechanism and in part, the same structural organization as other well-known P-ATPases such as calcium-transporting ATPases or sodium/potassium exchangers.
In 2011, the team led by Olivier Neyrolles at the Institute of Pharmacology and Structural Biology, Toulouse (France), highlighted a previously unknown role for P1B-ATPases. Firstly, this team showed that during infection by Mycobacterium tuberculosis, the pathogenic bacterium captured and stored in the phagosomes of the macrophage was subjected to toxic concentrations of zinc (
Figure A). She also showed that in order to survive this metal poisoning, the bacterium overproduced a P1B-ATPase, CtpC, whose role is to export excess zinc from the cytoplasm.
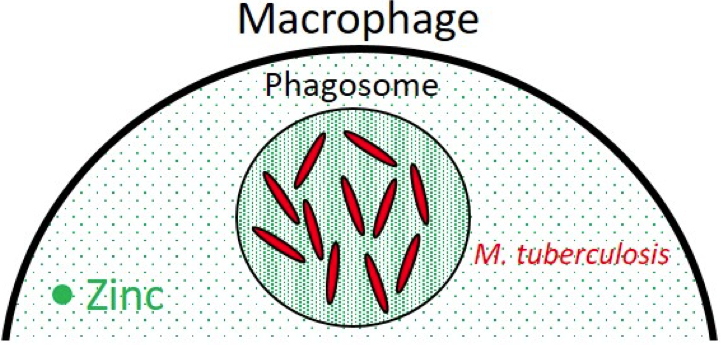 Figure A
Figure A:
M. tuberculosis inside the phagosome of a macrophage where zinc has accumulated. Credit CEA
In the present study, researchers from Irig [collaboration], experts in P1B-ATPases, show that CtpC cannot function without the presence of a small membrane protein of previously unknown function:
PacL1. This protein colocalizes with CtpC at microdomains in the bacterial membrane (
Figure B) and has a zinc binding motif at its C-terminus. Without PacL1, CtpC is no longer localized at the membrane and M. tuberculosis becomes highly sensitive to zinc. In this study, the researchers identified two other P1B-ATPases/PacL pairs in M. tuberculosis involved in metal transport: CtpG/PacL2 and CtpV/PacL3. In addition, other P1B-ATPases/PacL pairs are also found in different types of bacteria.
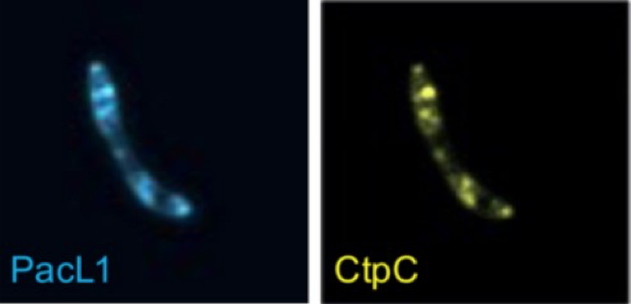 Figure B
Figure B: Epifluorescence image of PacL1
mTurquoise and CtpC
mVenus colocalisation in
M. tuberculosis. Credit CEA
This work suggests that metal resistance in some bacteria may use membrane platforms combining P1B-ATPases and small PacL-type chaperones, a new concept in the metallobiology of prokaryotes (
Figure C). They also open new perspectives for combating pathogenic bacteria by targeting their resistance mechanisms to metal stresses.
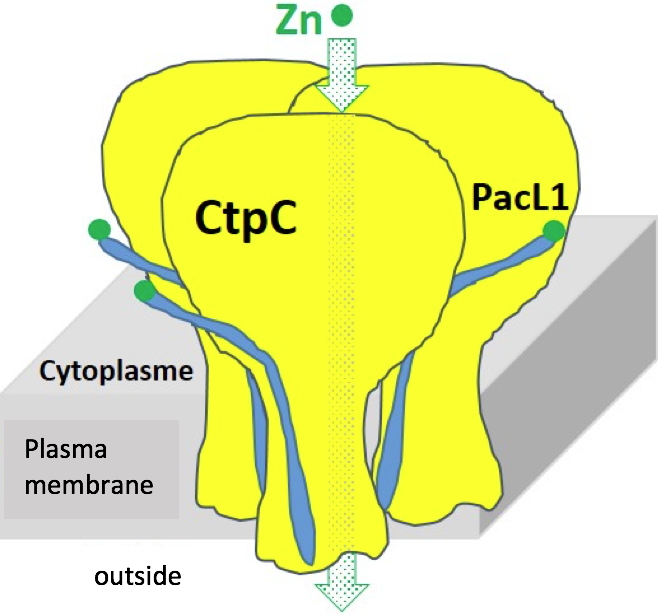 Figure C
Figure C: Schematic representation of a membrane platform for zinc efflux by CtpC/PacL1. Credit CEA
PacL1 (P-ATPase-associated
chaperone-Like protein
1) is a membrane protein required to the correct targeting and transport function of CtpC, hence the name "chaperone".