Electrolytes are central to technologies such as batteries, supercapacitors, and biosensors. At low concentrations, Debye–Hückel theory predicts that ions screen each other’s charges over a characteristic distance—the Debye length—which decreases as concentration increases. However, at high concentrations, Surface Force Balance (SFB) experiments have reported screening lengths unexpectedly growing with concentration, spanning tens or hundreds of ion diameters. This surprising result conflicts with simulations and theory, raising fundamental questions about electrostatic interactions in complex electrolytes.
Using large-scale molecular dynamics simulations of lithium tetrafluoroborate in ethylene carbonate, the authors systematically explored structural, dielectric, and transport properties across a broad concentration range. Direct comparison with SFB experiments revealed two distinct scales: a decreasing electrostatic screening length and a growing scale associated with the size of ionic clusters. The anomalous long-range decay observed experimentally is linked to these growing clusters, not to extended electrostatic forces, providing a consistent, parameter-free explanation.
This work reconciles a long-standing discrepancy between theory, simulations, and experiments by showing that anomalous screening reflects collective ionic clustering. Beyond solving a fundamental puzzle, these insights may guide the design of advanced electrolytes for next-generation energy storage and sensing technologies. This research was carried out in collaboration with the University of Ioannina, Greece. It was supported by the French National Research Agency through the France 2030 program (Grant ANR-22-PEBA-0002) and the MoveYourIon project (ANR-19-CE06/0025).
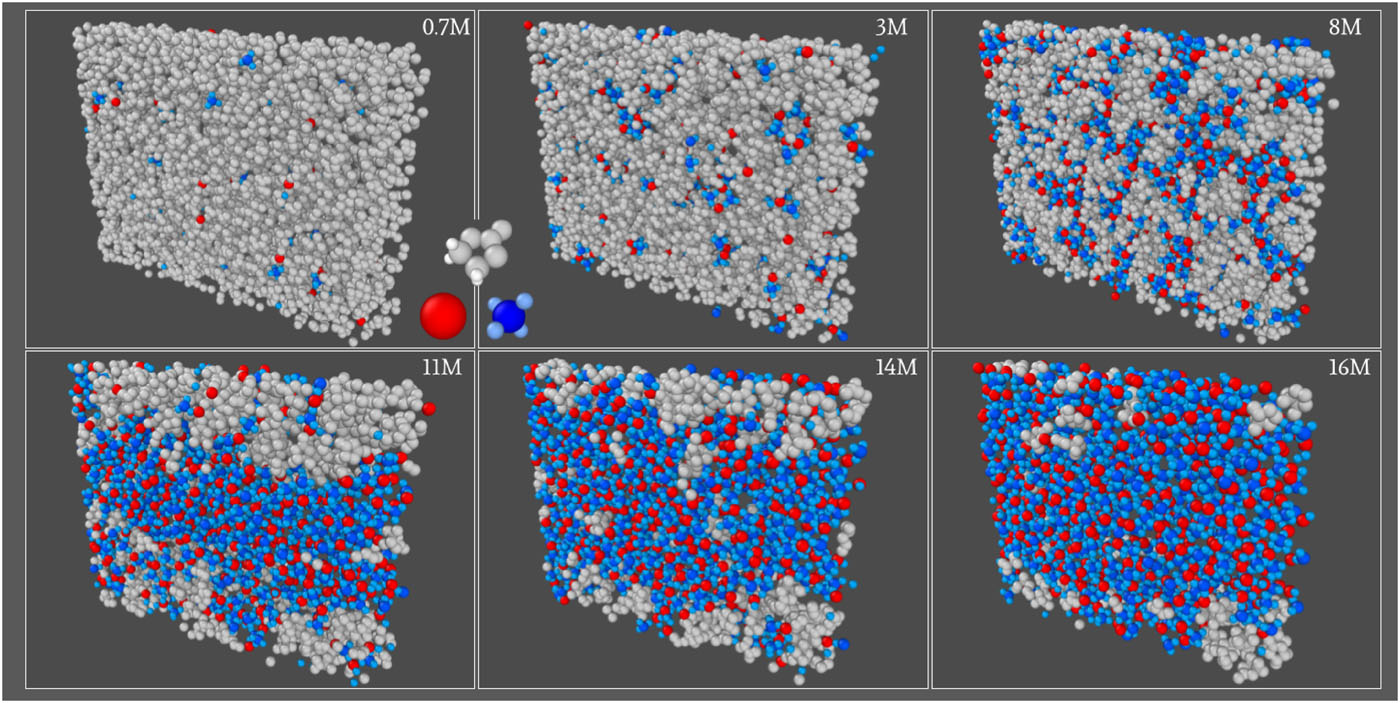
Figure: Representative MD snapshots show Li⁺ cations in red, BF₄⁻ anions in blue, and ethylene carbonate molecules in gray. As salt concentration increases, isolated ions merge into larger clusters, forming a percolated network and eventually phase-separating. © CEA