Recovering carbon waste resources as part of a circular economy
For many years, CEA has been conducting research on the conversion of biomass and carbonaceous waste to high calorific value gas as part of a circular carbon economy. Of the processes explored, hydrothermal gasification (HTG), or supercritical water gasification, is particularly notable for its ability to treat complex or wet materials without the need for a drying stage beforehand.
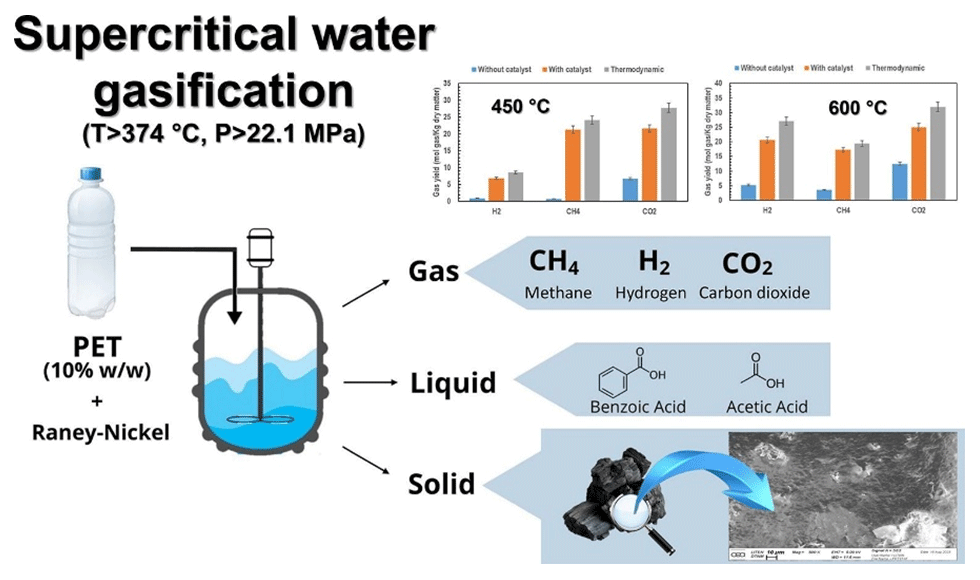
It is within this context that design and experimentation processes have been carried out on sewage sludge recovery equipment, highlighting a significant presence of plastics in waste treatment streams. This discovery has given rise to a specific line of research outside the initial framework, which aims to improve our understanding of the behavior of plastics under extreme hydrothermal gasification conditions (temperature > 374 °C; pressure > 221 bars). As part of this research, efforts have been focused on polyethylene terephthalate (PET), used in this case as a model compound representing a family of plastic waste present in treatment plant sludge. These works have led to the publication of a scientific article* explaining the conversion mechanisms observed in supercritical conditions.
Understanding the conversion of plastics in supercritical water
The study consisted in evaluating the conversion of PET via hydrothermal gasification in a reactor operating at temperatures higher than the critical point of water (temperature > 374 °C, pressure > 221 bars; e.g.: 450 °C and 600 °C) and at a pressure of 250 bars, with a dwell time of 30 minutes. The aim, on the one hand, was to produce an energy-rich gas, more specifically methane or hydrogen, and to identify recoverable molecules during the liquid phases on the other.
The findings showed that there was a significant increase in overall gas production as the temperature rose. Hydrogen was mostly produced at 600 °C, while methane was more commonly found at 450 °C. The addition of a Raney-nickel catalyst further optimised the conversion, increasing generation threefold at 600 °C compared to when the catalyst was not used, and also producing a high calorific value gas with a PCS of up to 64.7 MJ/Kg. Likewise, at 450 °C, greater performance levels with the catalyst were still observed than at a high temperature (600 °C) without the catalyst, suggesting lower operating temperatures would be best.
In addition, the aqueous phase obtained at 450 °C without a catalyst contains recoverable molecules such as benzoic acid and acetic acid, paving the way for chemical recycling.
Beyond this experimental performance, the study also drew on novel reaction pathways uncovered in the literature to explain the degradation of PET in such conditions. These breakthroughs have significantly enhanced our fundamental knowledge of the conversion of plastics via hydrothermal gasification.
Greater research prospects
These findings have brought about new possibilities for recovering non-recycled plastics, which are still difficult to treat via traditional procedures. A doctoral thesis was launched in late 2024 to better our understanding of conversion mechanisms, focusing on the thermoconversion and depolymerization of plastics in supercritical conditions. The initial results are promising and should lead, in the long term, to optimized operating conditions and uncover new thermochemical recovery methods.
Supercritical water gasification of plastic waste