Controlling the thickness of lithium metal a major challenge
In all-solid-state batteries, a solid electrolyte is used in place of today's liquid electrolytes. With promises of better performance, durability, and safety, this technology is one of the most eagerly-awaited breakthroughs in electrochemical energy storage and could respond to growing demand from the mobility and renewable energy industries.
For all-solid-state batteries to reach their full potential, a lithium metal (Li-metal) electrode must be used instead of the conventional graphite negative electrode. With the highest specific capacitance and lowest redox potential of all known electrode materials, Li-metal will allow to achieve significantly higher energy densities.
However, it is essential to be able to deposit very thin (less than 20 µm) of lithium, which is a prerequisite for:
- Maximizing cell energy density
- Reducing costs and environmental impacts
- Improving system safety
Today's rolling/calending processes cannot produce these very thin electrodes with the required regularity and repeatability.
Several CEA laboratories, Saft (a TotalEnergies company), and Automotive Cells Company (ACC) joined forces in 2022 to overcome this hurdle by developing an evaporation deposition process capable of producing thin (<20 µm), dense, and smooth lithium metal electrodes compatible with industrial-scale manufacturing.
Links between lithium metal thickness and three different failure regimes identified
Using characterization equipment adapted to the tricky analysis of Li metal at the CEA's Nanocharacterization Platform, researchers were first able to analyze in details the thin film properties deposited by evaporation at the CEA Tech regional center in Nouvelle-Aquitaine. These observations revealed very dense lithium layers with low roughness and low surface contamination.
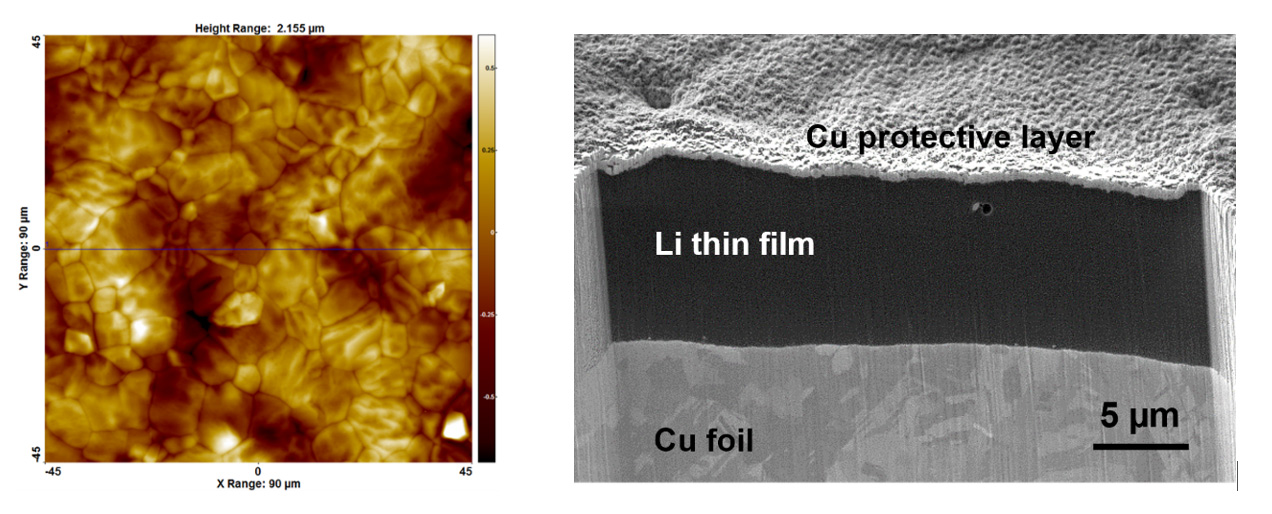
Left: An argon atomic force microscopy image of the surface of Li thin-film deposited by evaporation onto a copper foil. This image shows grain boundaries and Li grains emerging at the Li surface. The roughness obtained is very low, comparable to that of the underlying copper foil.
Right: A scanning electron microscopy image of a focused ion beam cross-section carried out at -140 °C on a Li thin film deposited by evaporation onto copper foil. It evidences the high density of the Li film.
The electrochemical responses of lithium metal electrodes of different thicknesses—from 2 µm to 135 µm—in a liquid electrolyte were thoroughly investigated to gain insights into the best tradeoff between the amount of lithium metal electrode and cell lifespan. For the first time in the literature, a link between lithium thickness and three different failure regimes was identified:
- In thin (<20 µm) electrodes, the low lithium content is the limiting factor, leading to premature cell failure.
- In thicker (>50 µm) electrodes, it is the increase of the resistance at the Li/electrolyte interface, due to irreversible lithium consumption, that leads to cell failure. Cycling performance doesn't depend on electrode thickness in this case.
- In electrodes between 20 µm and 50 µm thick, an intermediate behavior is observed, marking a clear transition between the two previous regimes.
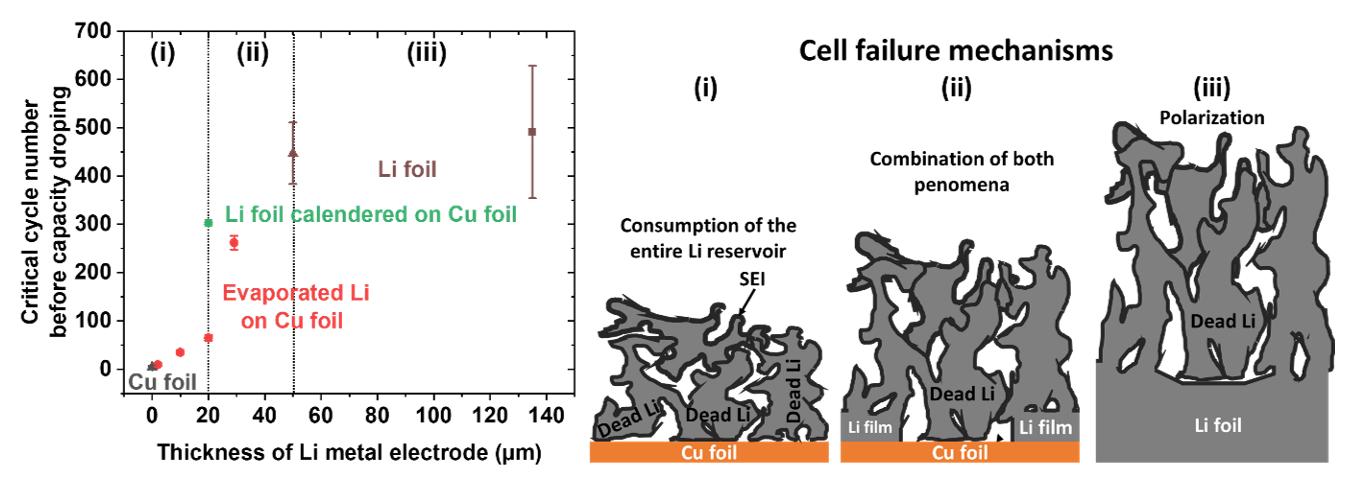
Highlighting of three battery failure mechanisms regarding the Li metal electrode thickness. A positive electrode made from LiNi0.8Mn0.1Co0.1O2 and a liquid electrolyte were used.
Taking the research further
CEA, Saft, and ACC combined their scientific and industrial expertise to develop a promising evaporation deposition process for producing high-quality and ultra-thin lithium metal electrodes.
Their work on the process, plus the insights gained into the failure regimes observed, will make this technology an even more credible choice for mobility, aerospace and defense. To plan the scale-up of this technology: the discovery of new breakthrough protective layers at the Li metal/electrolyte the interface will be needed to significantly extend the lifespans of lithium metal battery.