Turning CO
2 into methane using renewable electricity is a promising alternative to biogas production. This challenge was taken up by teams of researchers from the IRIG, the Department of Molecular Chemistry at the University of Grenoble Alpes, and the Indian association for the cultivation of science in Kolkata (India), who have just described a nickel-iron-based catalyst capable of carrying out this reaction. Directly inspired by the active site of enzymes, the catalysts of the living world, this unique compound has a selectivity comparable to the best metal-based materials described so far.
Methane, the main component of natural gas, is one of the major energy sources in our economy. To meet the objectives of the Paris Agreement, its extraction from fossil deposits will nevertheless have to be stopped by 2050. Hence the idea of producing this fuel directly from recycled CO
2 and renewable energies, and thus develop a circular carbon economy. But how can such a virtuous cycle be achieved?
Electrochemistry can provide the solution. The CO
2 molecule, long considered as waste, can be transformed into many products at the electrode surface depending on the number of protons and electrons brought to it. The most common and simplest reactions involve only two electrons and two protons to form, for example, synthesis gas or formic acid. It is also possible to produce methane from CO
2, but the reaction to obtain it directly is more complex, involving eight electrons and eight protons. Efficient catalyst for this multi-electronic process thus remains to be identified.
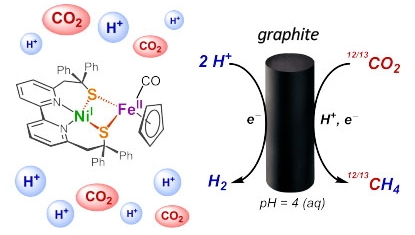
These teams just achieved it by using a complex based on nickel and iron, directly inspired by active sites of metalloenzymes involving these same metals in CO
2 metabolism. This complex has proven to be very efficient in catalytically converting CO
2 into methane. The selectivity of this molecular complex is unique for this complex reaction, even compared to the best catalytic materials described so far. To further improve the selectivity of methane production, the researchers are now trying to understand the mechanism at the origin of this chemical transformation.