Some bacteria are able to fix atmospheric nitrogen and convert it into ammonia at room temperature, a reaction of major importance for plants (nitrogen cycle). The enzyme responsible for this reduction is nitrogenase, a metalloprotein that uses two metal centers: P-cluster and FeMo-co. The first one is an atypical [Fe
8S
7] cluster, which allows the electron transfer to the FeMo-co. The latter is the active site itself and corresponds to an organometallic [MoFe
7S
9C-(R)-homocitrate] center. While the reaction mechanism of nitrogenase is still the subject of intense debate, the processes responsible for the FeMo-co production and insertion into the enzyme are far from being deciphered and are also sources of intense controversy.
The biosynthesis of FeMo-co requires the action of a dozen accessory proteins gathered in the NIF (NItrogen Fixation) assembly machinery. The NifB protein is the key enzyme in this process because it is responsible for the fusion of two [Fe
4S
4] centers and the insertion of a carbide ion and a sulfide ion to produce a [Fe
8S
9C] precursor called NifB-co. IRIG researchers are studying the catalytic mechanisms of metalloenzymes containing transition metal, but also the mechanisms of synthesis and insertion of these metal sites into the enzymes in which they are to be inserted. In 2020, in collaboration with the Universidad Politécnica of Madrid, they published the first crystal structure of the NifB protein, representing an early state of the NifB-co synthesis reaction. This work was quickly followed by another publication by a team of American researchers reporting a crystal structure with all metal centers present. Unfortunately, this team poorly modeled their crystallographic data and was therefore unable to correctly identify the active site content.
By revisiting these crystallographic data, the IRIG researchers were able to highlight the unexpected presence of a [Fe
8S
8] center resulting from the fusion of [Fe
4S
4] centers. This crystal structure allows to redefine the order of the reaction steps, showing that the fusion of the FeS centers must take place before the insertion of the carbide ion. The peculiar coordination of this intermediate clearly highlights the role of the protein matrix in the well-orchestration of the NifB-co biosynthetic steps, thus unveiling the mechanism of the enzyme.
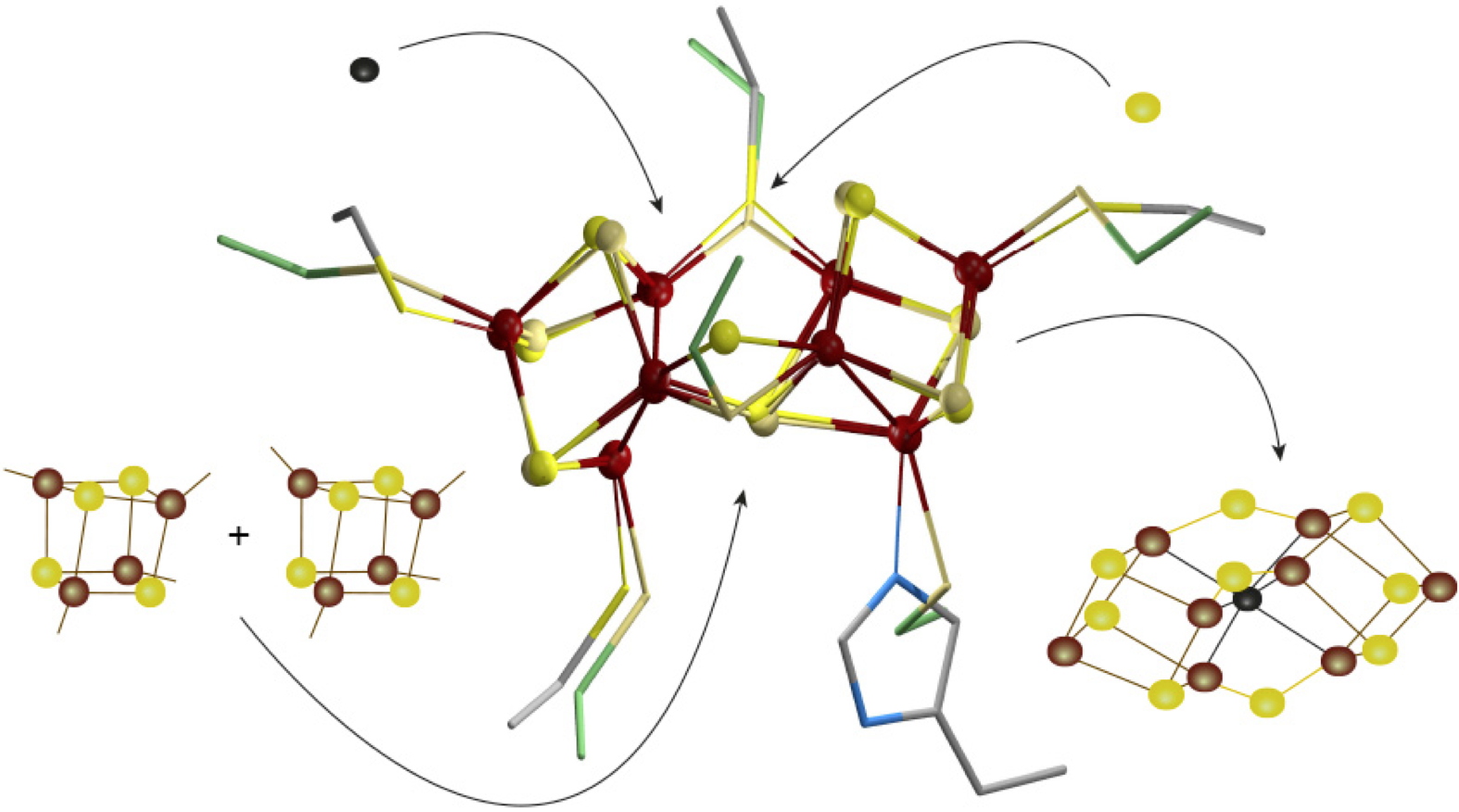
The K-cluster: a new intermediate in the synthesis of NifB-co, with a three-dimensional structure and coordination similar to that of the P-cluster. Schematic view of the different events allowing the fusion of two [Fe
4S
4] centers (left) leading to the K-cluster (in the center and superimposed on the crystal structure of the nitrogenase P-cluster), then the arrival of the carbide ion and the sulfide ion to produce NifB-co (right).