In a study published in Nature Structural and Molecular Biology, researchers from the CEA-IRIG/IBS, in collaboration with the CNRS/LCB (Laboratoire de chimie bactérienne de Marseille), looked at enzymes called oxidoreductases, which are essential for the conversion and transfer of energy in cells. These enzymes are often metalloproteins that contain one or more metal ions essential to their catalytic function, and appeared at the origins of life on Earth. Paradoxically, despite their diversity, these enzymes are actually based on a limited number of structural modules, the inventory of which is still far from complete.
By combining bioinformatics approaches with cutting-edge structural biology techniques, the scientists - collaboration CEA-Irig and CNRS-INSB - were able to identify a new structural module: a surprising tubular structure that connects oxidoreductases to the bacterial membrane and thus to quinones, small membrane molecules that are essential electrochemical connectors for energy transfer in cells. This structural model is described for a metalloenzyme involved in carbon metabolism in the soil bacterium Bacillus subtilis, catalysing the conversion of formate into carbon dioxide.
In summary, this study reveals a novel mechanism for anchoring oxidoreductases to the membrane, at the heart of microbial energy metabolism.
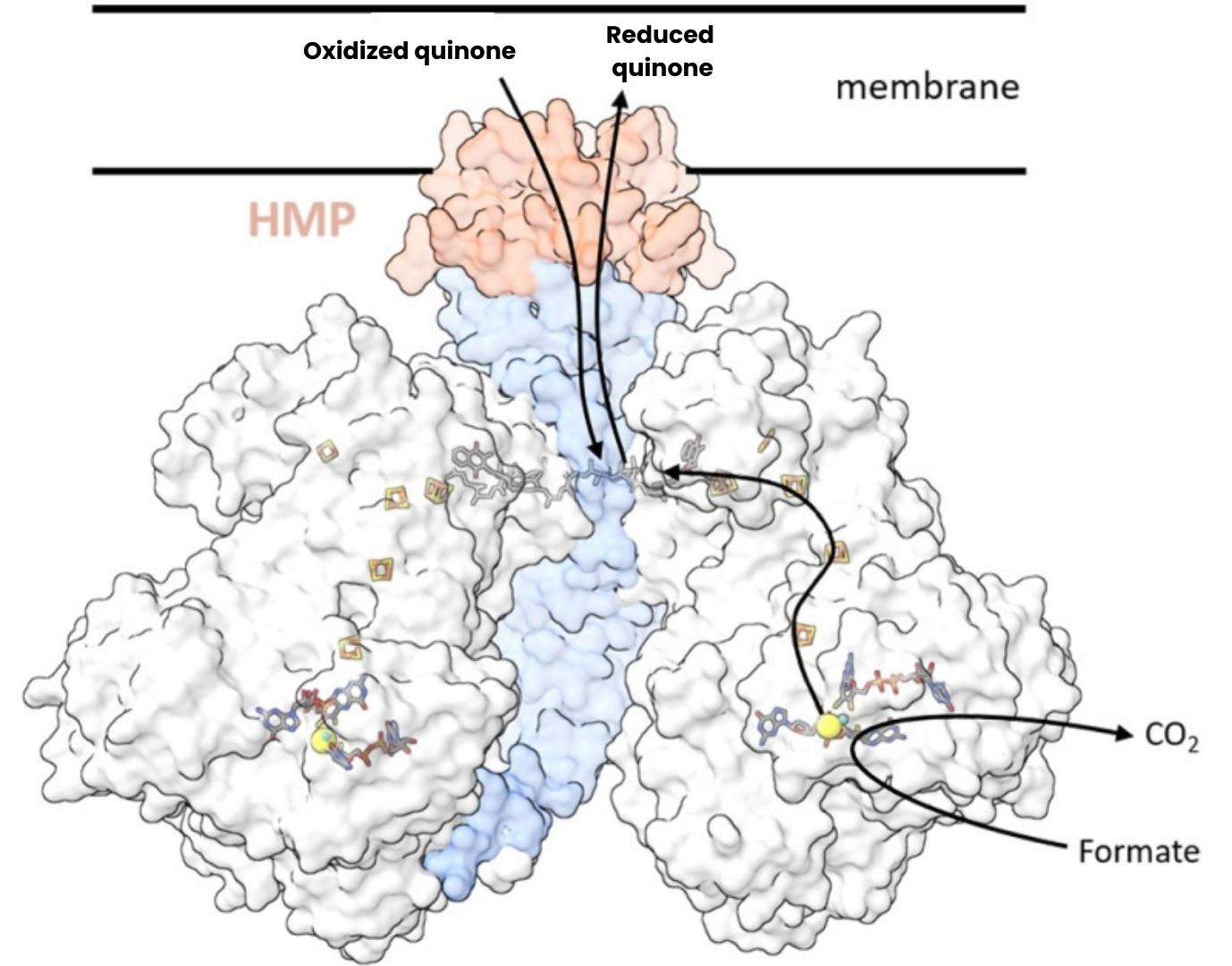
Figure: Structural organisation of the formate dehydrogenase complex in Bacillus subtilis revealed by cryo-electron microscopy. The catalytic subunits (in grey) are assembled around a central tube formed by four intertwined subunits (in blue) terminated by the HMP domain (in orange), providing the link with the membrane and the quinones. Bioinformatics analyses reveal that this type of architecture is shared by at least three other families of oxidoreductases in various micro-organisms.