Nafion membranes are central to fuel cell technology, yet their production remains costly. Furthermore, the use of fluorinated polymers raises environmental and health concerns, and no industrial recycling process currently exists, which constitutes a major challenge. This study proposes an innovative approach using ionic liquids (non-volatile liquid salts, composed entirely of ions, whose properties can be tuned for specific application) to disperse and subsequently recover the membranes. A multi-scale analysis, including macroscopic swelling kinetics, neutron and X-ray scattering, allowed us to decipher the swelling mechanisms driving membrane dispersion and to identify the most promising candidates for sustainable recycling.
We show that Nafion membranes can be dispersed in ionic liquids and subsequently recovered by precipitation and washing. The membranes are restored to their solid form, providing a first proof of concept for closed-loop recycling using ionic liquids as solvents. A multi-scale approach, combining swelling kinetics, light, neutron and X-ray scattering enabled us to elucidate the nature and sequence of interactions from the early stages of swelling to complete dispersion—including swelling of ionic domains, dilution of polymer aggregates and plasticization — and to identify the most promising families of ionic liquids for recycling.
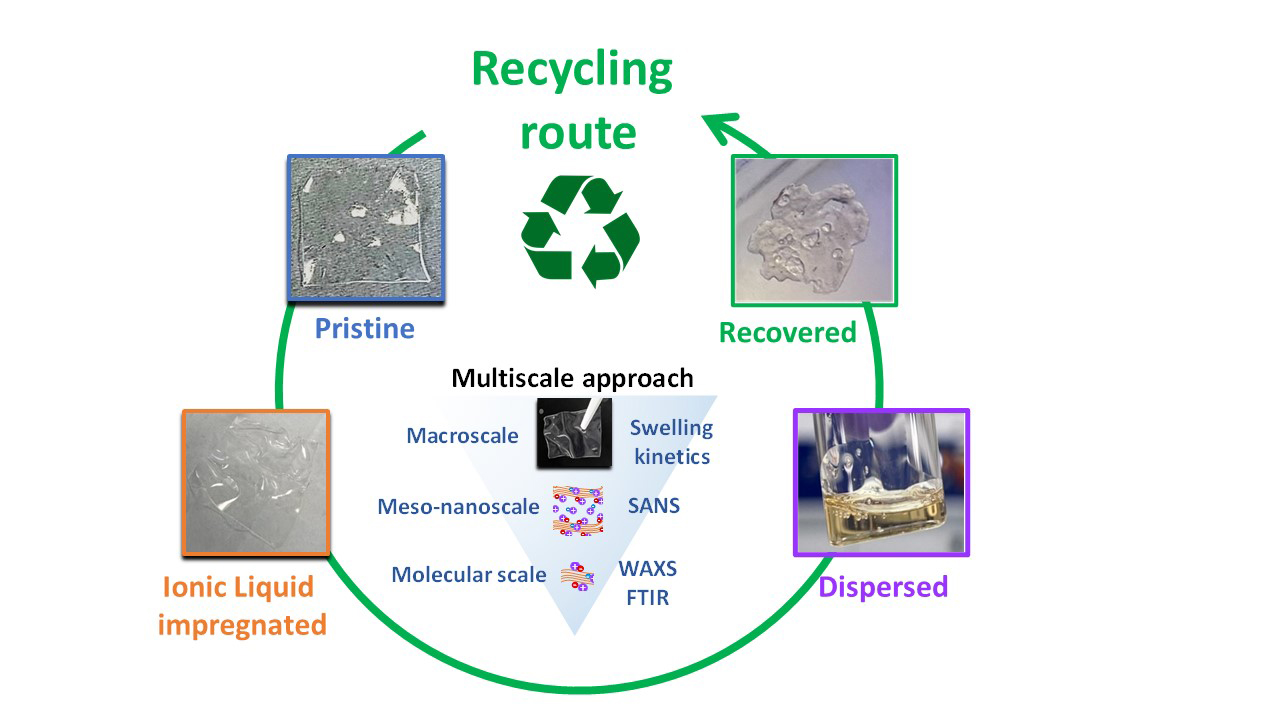
Figure : A new route for recycling Nafion in an ionic liquid medium
© CEA-Irig/SyMMES/ Hakima Mendil-Jakani
This study establishes a proof of concept for recycling Nafion in an ionic liquid environment and complements recent work on novel recycling methods for membrane-electrode assemblies from fuel cells. Future work will aim to extend this process to aged membranes or alternatives to Nafion.
fuel cell*: electrochemical device that produces electricity by converting chemical energy from a fuel into electrical energy, through reactions occurring at the electrodes and the movement of protons across an electrolyte (the membrane).
Nafion*: fluorinated polymer used as a proton exchange membrane in fuel cells, widely used for its electrochemical performance.
swelling kinetics*: measurement of the variation in mass and volume of a material over time due to liquid absorption.
UMR : SyMMES, Univ. Grenoble Alpes, CEA, CNRS, Grenoble INP - UGA
Funding : PTC MP
Collaborations :
Univ. Grenoble Alpes, CEA, LITEN, DTNM, 38000 Grenoble, France
Univ. Grenoble Alpes, CEA, IRIG, MEM, 38000 Grenoble, France
Institut Laue-Langevin, 38000 Grenoble, France
ICGM, University of Montpellier, CNRS, ENSCM, Montpellier, France
Tribute This article is dedicated to the memory of our co-author, Gérard Gebel, whose recognized expertise in the structural analysis of ionomer membranes greatly enriched the scientific environment of this work. His wise advice during Ana Carolina's thesis was invaluable. Gérard passed away before the publication of this article; we honor his memory and scientific legacy with respect and gratitude.