Heparan sulfates are complex
polysaccharides belonging to the
glycosaminoglycan (GAG) family, abundantly present on the cell surface and in
extracellular matrices. They are in a branched form on glycoproteins that decorate the cell surface and are involved in many physiological and pathological processes. Their structure and their ability to bind and modulate the activity of a very large number of proteins are finely controlled, in particular by enzymes such as HSulf-2 endosulfatase, secreted in the extracellular environment, which catalyzes a targeted desulfation of the polysaccharide. The understanding of the mechanisms involved in these regulations represents an obvious challenge for the study of many cellular functions.
However, endosulfatases remain extremely enigmatic enzymes. Thus, their molecular structure, catalytic mechanisms and substrate specificity are still poorly documented, and their study has led to an abundance of contradictory data (discrepancies between in vitro and in vivo data depending on the biological system, isoforms of enzymes showing anti-oncogenic or pro-oncogenic activities...).
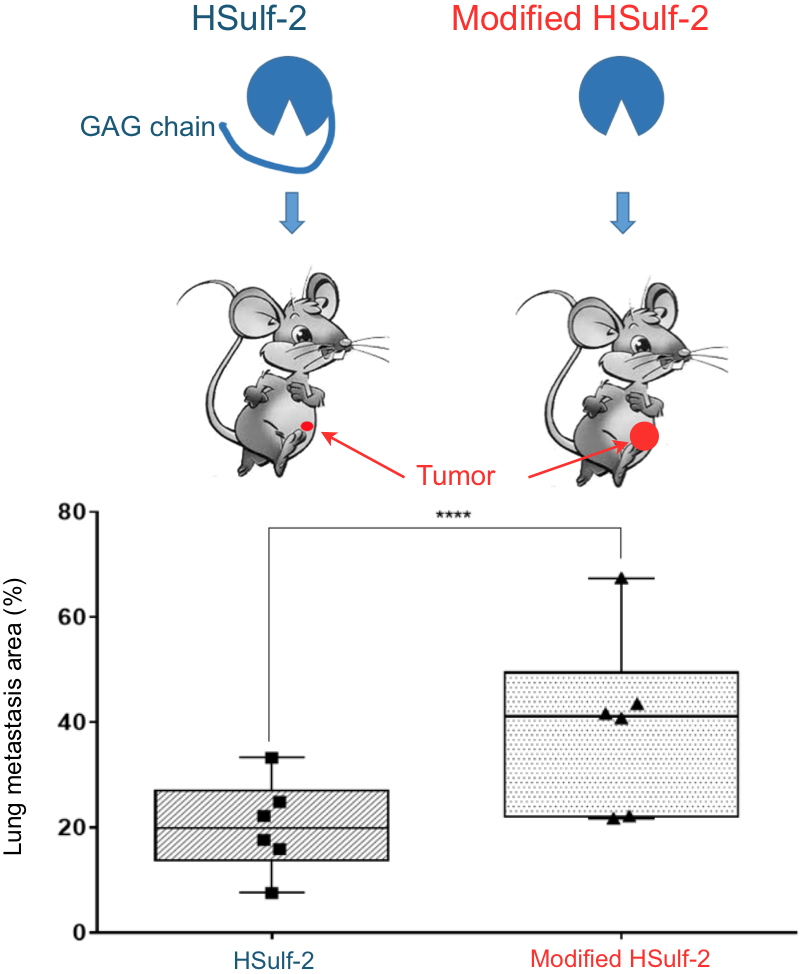
HSulf-2 is one of these enzymes. It is being studied by IRIG researchers who have just shown that HSulf-2 itself carries a GAG chain. They have analyzed the effect of this chain in various
in vitro and cellular assays, as well as in an
in vivo cancer xenograft model in mice. Using a mutant of HSulf-2 lacking its GAG chain, the researchers showed a significant increase in the activity of the enzyme
in vitro. The expression of this mutant in mammary cancer cells promotes cell proliferation, migration and invasion
in vitro, but also tumor growth and the appearance of pulmonary metastases in an
in vivo model (
Figure. Credit CEA). They also showed that a family of glycosidases, the hyaluronidases, could release the enzyme from its GAG chain and restore its activity.
These data clearly show that the GAG chain of HSulf-2 acts as a modulator of the pro-tumor activity of the enzyme, and that the degree of "GAGosylation" of HSulf-2 is an important factor to consider when studying the role of the enzyme in extracellular matrix remodeling. This discovery highlights a novel regulatory process and may help clarify nearly two decades of conflicting data in the study of endosulfatases. Finally, it opens a new field of investigation to try to fight primary and metastatic cancer by targeting HSulf-2.
Polysaccharides (sometimes called glycans or complex carbohydrates) are polymers of the carbohydrate family. The most common and well-known in the plant kingdom are cellulose and starch, both polymers of glucose.
Glycosaminoglycans (GAGs) are polysaccharides of animal origin, found in abundance on the surface of cells and in the extracellular matrices of the conjunctive tissue. Most GAGs are long linear sulfated chains covalently associated with specific glycoproteins, and are capable of interacting with and modulating the activity of hundreds of protein ligands (structural, signaling, adhesion proteins, enzymes...).
The extracellular matrix is an assembly of macromolecules (collagens, elastin and structural glycoproteins) that bind cells and organize them into tissue.