Today, the development of new catalysts that are both high-performing and environmentally friendly is essential as sustainable alternatives to certain industrial processes. A notable example is the water-gas shift reaction (WGSR, CO + H₂O ↔ CO₂ + H₂) which traditionally relies on industrial catalysts operating under high pressure and temperature. This reaction, used to enrich syngas (produced from petrochemical processes) in hydrogen for the synthesis of liquid fuels, also occurs naturally in certain bacteria. In these microorganisms, the reaction is catalyzed under mild conditions by two enzymes: a carbon monoxide dehydrogenase (CODH) and a hydrogenase. The objective of the
CEA-IRIG/LCBM Team is thus to develop efficient and innovative enzymatic systems.
Syngas, composed primarily of H₂, CO, and CO₂, holds significant potential for the production of liquid fuels and chemical intermediates. It serves as a versatile feedstock in processes such as Fischer–Tropsch synthesis, where the H₂/CO ratio plays a critical role in determining the efficiency and selectivity of the reactions. The WGSR enables adjustment of this ratio by converting CO and water into CO₂ and H₂, thereby optimizing syngas composition for specific applications. For instance, methanol synthesis requires a 1:2 CO/H₂ ratio, while the typical ratio in raw syngas is approximately 0.3. The WGSR proceeds via two half-reactions: oxidation of CO (CO + H₂O → CO₂ + 2e⁻ + 2H⁺) and proton reduction to H₂ (2e⁻ + 2H⁺ → H₂).
The aim of this work was to design a bicatalytic system combining an enzyme and a molecular complex: a carbon monoxide dehydrogenase (CODH), capable of oxidizing CO to CO₂, coupled with a nickel-based bioinspired catalyst that efficiently reduces protons to H₂. The CODH was first characterized at the molecular level, then integrated into a biohybrid system in which both catalysts are immobilized on carbon nanotubes. These nanotubes play a key role by facilitating electron transfer between the two catalysts, thereby accelerating CO conversion to CO₂ and concurrent hydrogen production (see
figure).
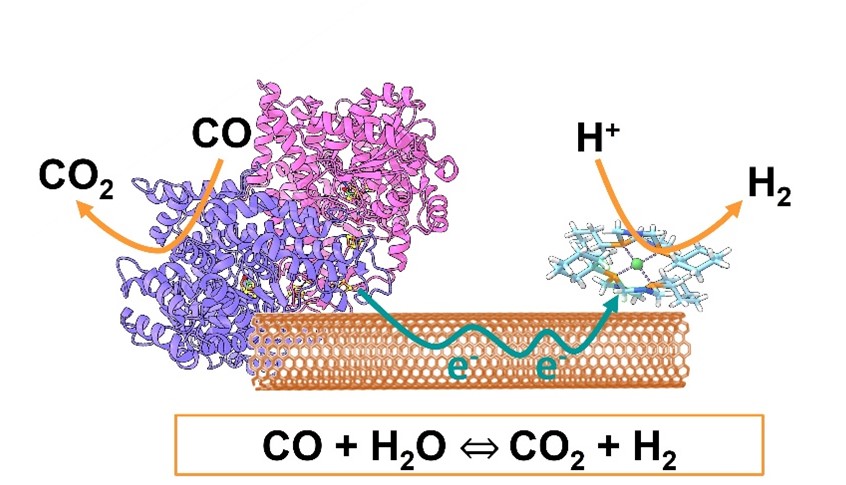
Figure: biohybrid system for the WGSR combining an enzyme, a bioinspired complex and carbon nanotubes. © CEA
Thanks to the high CO tolerance of the nickel complex, this innovative biohybrid system demonstrates excellent performance. It achieves complete conversion (100%) and a maximum reaction rate of 30 s⁻¹ for the WGSR, under ambient temperature and pressure conditions, without any external energy input, whether using pure CO or synthetic gas as the substrate.
The system developed in this study represents a first proof of concept. One of the major challenges lies in the development of Power-to-Syngas processes, which aim to produce highly pure syngas from water, CO₂, and renewable energy by optimizing the reaction in the reverse direction. For example, water electrolysis can be coupled with the reverse water-gas shift reaction (CO₂ + H₂ → CO + H₂O). In this context, optimizing the WGSR —whether in its forward or reverse direction — emerges as a promising research avenue in catalysis for sustainable chemistry. It opens new perspectives for the development of CO₂, biomass, and waste valorization processes, aligned with the goals of a circular carbon economy.
Fundings
- Programme NTE CEA
- Programme ECC CEA
- PEPR Spleen Projet POWERCO2
Collaborations
- Département de Chimie Moléculaire UGA,Grenoble, France
- Laboratoire de Bioénergétique et Ingénierie des protéines, CNRS Marseille, France