In our OLED (Organic Light-Emitting Diode) screens, the quality of the light produced is greatly diminished by the anti-reflection filters that equip them (Scheme). Indeed, these filters prevent the reflection of external light (sunlight, street lighting) on the OLED slab, but if the device does not emit circularly polarized light (see paragraph below), 50% of the light intensity produced by the diode is lost.
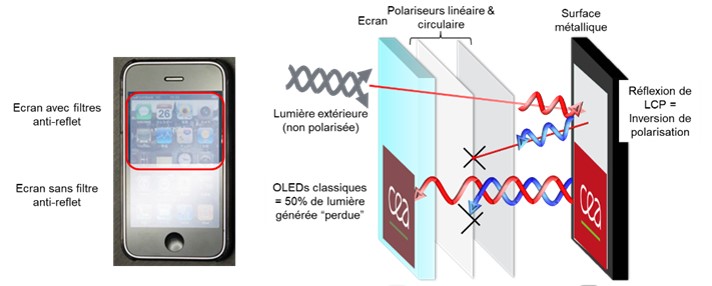
Scheme of the phenomenon decreasing the efficiency of OLED screens because of their anti-reflection filters © L. Favereau (CNRS/University of Rennes)
In 2016, work conducted by SCBM researchers (Feuillastre et al, JACS, 2016) led to the development of purely organic electroluminescent materials (without rare metals) which, through their design and structure, exploit three properties that improve their "energy consumed" versus "quality of light produced" performance : i) the emission of Thermally Activated Delayed Fluorescence (TADF), which theoretically makes it possible to transform all electrical energy into light ; ii) the increase of light emission by aggregation or how designed luminophores still gain in efficiency when their concentration is increased and they are arranged in solid material, contrary to classical luminophores which see their efficiency drop at this stage (Figure 1) ; iii) the emission of circularly polarized luminescence (CPL)*, which passes through the anti-reflection filters placed on the screens without attenuating the light produced by the OLED slab, a very rare phenomenon in the organic world.

Figure 1 : Illustration of the light gain of the new luminophores when they aggregate (from left to right: increase in concentration) © L. Frédéric (SCBM/CEA)
While classical CPL-emitting fluorophores are intrinsically chiral (high synthesis cost), the concept of "chiral perturbation" makes it possible to confer chiroptic properties to an active chromophore by attaching to it a commercially available chiral unit** that already has the desired spatial geometry (Figure 2). In the present study, researchers have synthesized a series of novel luminophores to perform the first systematic analysis of the structure/properties relationships for this type of organic materials and to understand the parameters influencing the efficiency of the chiral perturbation and thus the obtention of the best properties for these materials. Some of the obtained molecules were then integrated into the first circularly polarized light emitting OLEDs, architecturally designed to fit into high-resolution portable displays.

Figure 2 : Chemical formulation of the new chromophores, integrating the 3 properties
allowing to improve the ratio "quantity of energy consumed / quality of light produced" in display systems. G. Pieters (SCBM/CEA)
Optimizing the phenomenon of chiral perturbation was essential so that the teams could continue to work on improving the quality of the light produced and obtain a true OLED material that is revolutionary and exploitable for the industry.
* Circularly Polarized Luminescence : The polarization of light is assimilated to the ordering of the photons that make up the luminous flux: in a "classical" flux, photons do not have an ordered movement, contrary to the polarized flux. "Circularly polarized" means that the photons of the luminous flux follow the same circular trajectory.
** Chirality : the same molecule can have different spatial configurations. Like our hands, some molecules have possible configurations that mirror each other: these molecules are called "chiral". These configuration changes can impact their physical properties or their interaction with the living (example: drugs).
News CEA/DRF