About ten years ago, a team from IPMC* discovered in black mamba venom Mambalgin-1 (Mamb-1), an analgesic peptide in rodents, as powerful as morphine but with less undesirable side effects, the opioid-independent mode of action of which involves specific inhibition of ASICs (Acid-Sensing Ion Channels). ASICs are voltage independent cationic neural channels that primarily conduct Na+ ions and are activated by extracellular acidification and lipids. Widely expressed in the peripheral and central nervous systems, and involved, among others, in the processes of nociception and neuronal plasticity, they constitute interesting targets for clinical applications in the field of pain, psychiatry, neurodegenerative diseases and cancer.
Since the discovery of Mamb-1, the IPMC team has been working with SIMoS (DMTS) to elucidate its mode of action. Indeed, understanding in detail how Mamb-1 inhibits mammalian ASIC will allow the development of optimized blockers of these channels. Numerous studies, including the recent low-resolution cryo-EM structure of ASIC1-Mamb-1 complexes, have shown that Mamb-1 inhibits the ASIC1, by binding preferentially, on the extracellular side, to the closed state of this channel, that it stabilizes. Interestingly, these structures, even if they do not provide a detailed description of the interaction between the peptide and the channel, contradict the model proposed by our teams in 2016, according to which Mamb-1 would bind deeply the pH-sensitive pocket of the channel.
In this context, IPMC and SIMoS researchers took advantage of the recent publication of the closed state crystallographic structure of chicken ASIC1 to model the interaction between the rat ASIC1 channel and Mamb-1, whose 3D structure was solved by these teams in 2016. For this, they chemically synthesized different variants of Mamb-1 and studied their interaction with variants of the rat ASIC1. Analysis of the results identified pairs of potentially interacting residues of Mamb-1 and the channel, which were used to model, by molecular dynamics, the complex between Mamb-1 and the rat ASIC1 in its closed state. The detailed image of the interactions thus obtained (Figure), compatible with the structure of the Mamb-1 / human ASIC1 complex obtained by low-resolution cryo-EM, makes it possible to propose that Mamb-1 does not bind directly to the pH sensor of the channel but locks by several contacts a key hinge between the α4 and α5 helices of an extracellular domain of ASIC1 to prevent the opening of the channel.
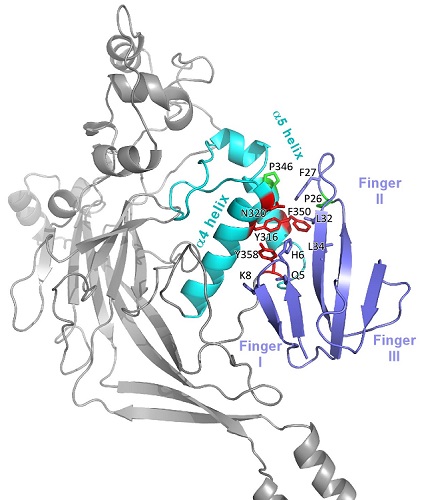
Structural model of the complex between Mamb-1 and rat ASIC1.
Zoom on the interaction region involving the α4 and α5 helices of ASIC1 (in cyan) and the fingers 1 and 2 of Mambalgin-1 (in blue). The major residues in this interaction are shown.
In conclusion, these results provide an improved model of inhibition of mammalian ASIC1 by Mamb-1 and clues for the further development of optimized ASIC blockers.
Contact : Denis Servent
* IPMC : Institut de Pharmacologie Moléculaire et Cellulaire, UMR7275, Université Côte d'Azur, CNRS.