BIOTHERAPEUTICS : REACHING THE BRAIN THROUGH THE NASAL ROUTE
Trastuzumab (TZB) is a therapeutic antibody that has significantly improved the survival of patients with HER2-positive breast cancer. However, the incidence of brain metastases in these patients is rising and associated with poor prognosis. The limited efficacy of systemically injected biotherapeutics is explained by their extremely low distribution into the central nervous system (CNS), largely due to the BBB, which tightly regulates molecular exchanges between the blood and the brain. This limited permeability poses a major challenge for the treatment of brain tumors.
Nose-to-brain (N2B) administration, which bypasses the BBB, represents a promising alternative for treating these tumors. Research has focused on biodegradable and biocompatible materials, such as nanogels and functional nanoparticles (NP), capable of transporting drugs from the nasal cavity to the CNS. Transport is made possible by the direct anatomical connections between the nasal cavity and the brain via the olfactory and trigeminal nerves.
EXAMPLE OF TRASTUZUMAB IN A NASAL EPITHELIAL MODEL
After characterizing the NP-TZB formulation (spherical nanoparticles around 200 nm in diameter with a 67% TZB encapsulation efficiency), the researchers demonstrated successful transport across a nasal epithelial model. They also compared the transport of NP-TZB with that of free TZB across an in vitro BBB model simulating systemic administration. NP-TZB significantly increased TZB transport across the nasal epithelial barrier, whereas no significant improvement was observed across the in vitro BBB model. The nanoparticle-transported TZB released into the basal compartment remained fully functional and retained its ability to recognize HER2 expressed on the surface of breast cancer cells (BT474).
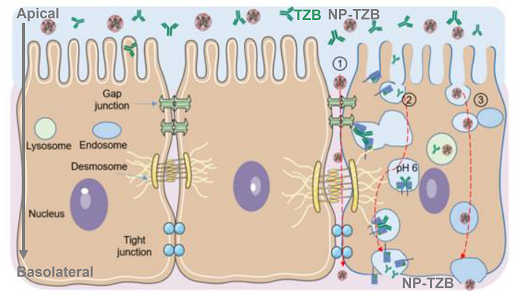
Hypothetical mechanisms of TZB (green) and NP-TZB (pink) transport across the nasal epithelial barrier model (RPMI 2650). © Kengne Kamkui et al., Pharmaceutics 2025
Following this first in vitro validation step, the next stages will involve assessing the in vivo biodistribution of NP-TZB and then evaluating the potential therapeutic efficacy of N2B delivery in a preclinical model.
Joliot contacts : Aloïse Mabondzo (aloise.mabondzo@cea.fr) ; Didier Boquet (didier.boquet@cea.fr)
- Trastuzumab is a recombinant humanized monoclonal antibody that inhibits the proliferation of human tumor cells overexpressing HER2 (breast cancer).
- Poly(lactic-co-glycolic acid) (PLGA) is a biocompatible aliphatic polyester and a widely used biodegradable polymer. FDA-approved, PLGA has long been employed for drug delivery in humans. Hydrophobic and hydrophilic drugs can be encapsulated into PLGA particles via single or double emulsion techniques.