KIF2C : a key player in the mitotic dynamics of microtubules
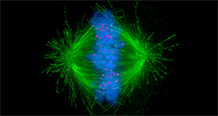
During mitosis, the accurate distribution of chromosomes into the two daughter cells is a highly controlled process essential for genome integrity. During this phase, microtubules reorganize into a bipolar spindle. The microtubule depolymerase KIF2C plays a key role in microtubule dynamics. It facilitates attachment between chromosomes and microtubules, thereby contributing to correct chromosome segregation and genome stability during cell division. Dysregulation of KIF2C has been observed in several pathologies, including various cancers, as well as during normal and pathological aging. In some breast cancers, KIF2C overexpression leads to the depolymerization of microtubules stabilized by paclitaxel, contributing to cellular resistance to this drug.
It has already been shown that KIF2C forms foci in mitotic cells and that some of these foci co-localize with kinetochores. A collaborative study led by Sophie Zinn-Justin and the IGH[1], involving the Institut Curie, ENS, CEA-Jacob, and two European teams, revealed that KIF2C forms membrane-less organelles (or condensates) at the surface of microtubules, particularly at kinetochores. These condensates concentrate kinases, including a central mitotic kinase, PLK1, which contributes to KIF2C activation.
Furthermore, the researchers show, using optogenetic experiments, that KIF2C condensates form through interactions mediated by its N-terminal domain. Unexpectedly, they found that this N-terminal domain adopts a Tudor/PWWP/MBT-like fold that binds to phosphorylated motifs. In particular, KIF2C directly interacts with the DNA damage repair protein BRCA2 phosphorylated by PLK1 on T207 (BRCA2-pT207).
The authors also demonstrate that KIF2C condensation :
-
does not depend on BRCA2-pT207, but requires active Aurora B and PLK1 kinases,
-
concentrates PLK1 and BRCA2-pT207 in an Aurora B–dependent manner,
- is promoted by the depolymerase activity of KIF2C,
- excludes tubulin: KIF2C interacts with tubulin only at the periphery of condensates, which are therefore located on/next to microtubules, particularly at their ends.
Altogether, the results suggest that, during kinetochore–microtubule attachment, the assembly of KIF2C condensates amplifies the catalytic activities of PLK1 and KIF2C, and spatially concentrates BRCA2-pT207 at microtubule ends. The researchers propose that this newly described and finely regulated mechanism contributes to the control of kinetochore–microtubule attachment, chromosome alignment, and their stability.
Contact at I2BC / Frédéric-Joliot Institute for Life Sciences:
[1] Team "Instabilité génétique et cancer" at the Institut de génétique humaine, Montpellier.
 Carousel image:
Carousel image:
-
Author: Afungy at English Wikipedia.
-
Source: https://en.wikipedia.org/wiki/File%3AKinetochore.jpg