2,5-diketopiperazines (2,5-DKP), produced by fungi, bacteria, plants and animals, have properties (antibacterial, antiviral, anticancer or anti-inflammatory) that make them very attractive for pharmacopoeia. The I2BC team (SBIGeM) has been studying for several years the bacterial biosynthetic pathways of these molecules and exploits them, in collaboration with teams from the Sorbonne University, to develop synthetic biology processes to produce 2,5-DKP in the chassis bacterium Escherichia coli.
2,5-DKPs are synthesized in several steps: cyclodipeptide synthases (CDPS) make cyclodipeptides from the amino acids loaded on the transfer RNAs (tRNAs), which are then modified by other enzymes to form 2,5-DKPs with remarkable biological properties. In the present study, the team focused on cyclodipeptide oxidases (CDOs). CDOs are involved early in the biosynthesis pathways of 2,5-DKPs and contribute to their chemical diversification by producing dehydrogenated 2,5-DKPs via a Ca-Cb dehydrogenation reaction. The objective of this study is to provide a toolbox for efficient bio-production in E coli of dehydrogenated 2,5-DKPs.
To do this, researchers first analyzed bacterial genomes in order to identify genes coding for CDOs in the 2,5-DKP biosynthesis pathways. Six of these genes, corresponding to CDOs of unknown activity, were co-expressed in E coli with those coding for the associated CDPS. The chemical structure of the dehydrogenated cyclodipeptides thus produced in the culture supernatants was resolved to characterize the enzymatic activity of the studied CDOs. In a second step, in order to optimize the bio-production of these dehydrogenated 2,5-DKP in E coli, combinatorial engineering was carried out : each of the genes coding for the six CDOs studied as well as for two previously characterized CDOs was co-expressed with one of the eighteen genes coding for a CDPS. Among the 144 combinations, the best CDO/CDPS pairs (allowing the production of the highest levels of many dehydrogenated 2,5-DKPs) were identified.
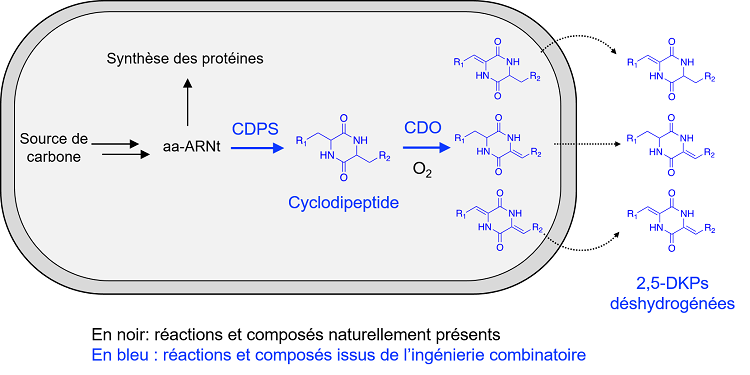
Schematic representation of an E coli bacterium programmed for the production of dehydrogenated diketopiperazines. CDPS uses tRNA-charged amino acids (aa-RNA) to produce cyclodipeptides that are then dehydrogenated by CDOs. Dehydrogenated 2,5-DKPs are finally recovered from the culture medium. R1 and R2 represent chemical groups characteristic of amino acid side chains.
This study establishes the usefulness of CDPS and CDOs for the bio-production of many dehydrogenated 2,5-DKPs in
Escherichia coli. It is the first step towards the bio-production of more complex products.