WHAT IS AN IRON-SULFUR CENTRE ?
Iron–sulfur (Fe–S) clusters are assemblies of iron and sulfide that form the active sites of a large number of proteins involved in essential biological processes, including ATP production, enzymatic catalysis, protein synthesis, and genome integrity maintenance. These cofactors are synthesized by multi-protein machineries and subsequently inserted into target proteins through a complex, multi-step process. Deficiency in any component of this machinery can result in severe diseases, the most common in humans being Friedreich's ataxia (FRDA), a rare neurodegenerative and cardiac disorder caused by a genetic deficiency in frataxin. Therapeutic strategies based on compounds mimicking frataxin function remain limited by incomplete knowledge of the mechanisms governing Fe–S cluster biogenesis. Moreover, gene therapy approaches are challenged by the toxicity associated with frataxin overexpression.
For several years, Benoît D'Autréaux's team has been working to elucidate the biosynthesis mechanism of these clusters by reconstituting the iron–sulfur cluster (ISC) assembly machinery in vitro. In 2022, they demonstrated that Fe–S cluster biosynthesis is a highly conserved process, initiated by iron insertion at the assembly site of the scaffold protein IscU (see Joliot news). In 2025, the authors published two landmark studies.
STEP-BY-STEP ASSEMBLY OF [2FE–2S] CLUSTERS
In the first study, the researchers dissected the assembly process of a [2Fe–2S] cluster step by step using an in vitro reconstituted system with proteins from Escherichia coli, combined with biochemical and spectroscopic techniques. They showed that assembly proceeds sequentially on the IscU scaffold protein: iron binds first, followed by sulfur in the form of a persulfide provided by the cysteine desulfurase IscS. Electrons are then supplied by a ferredoxin (Fdx) to cleave the persulfide into sulfide, leading to the formation of a [1Fe–1S] precursor. Subsequently, a [2Fe–2S] cluster is formed through IscU dimerization and fusion of two [1Fe–1S] precursors.
A FINELY TUNED REGULATORY MECHANISM INVOLVING FRATAXIN AND FERREDOXIN-2
In the second study, the team uncovered a complex regulatory mechanism governing human [2Fe–2S] cluster biosynthesis involving ferredoxin-2 (Fdx2) and frataxin (Fxn). The assembly process is similar to that observed in bacteria: iron binds to the assembly platform Iscu2, followed by sulfur in the form of a persulfide supplied by the desulfurase Nfs1. This persulfide is then cleaved into sulfide by Fdx2, resulting in the formation of a [2Fe–2S] cluster. Frataxin stimulates the entire process by accelerating persulfide transfer between Nfs1 and Iscu2. Using an in vitro reconstituted human ISC system, the authors show that cluster synthesis is optimal when Fxn and Fdx2 concentrations are nearly equal. Any deviation from this balance reduces reaction efficiency. This effect arises from competition between Fxn and Fdx2 for the same binding site on the Nfs1–Iscu2 complex.
Based on their in vitro findings, the researchers hypothesized that reducing Fdx2 levels in a context of frataxin deficiency (Friedreich's ataxia) could improve Fe–S cluster biogenesis in vivo. Using Drosophila as a model of FRDA (reduced frataxin levels), they observed that decreasing Fdx2 expression increased fly lifespan, thereby validating their hypothesis.
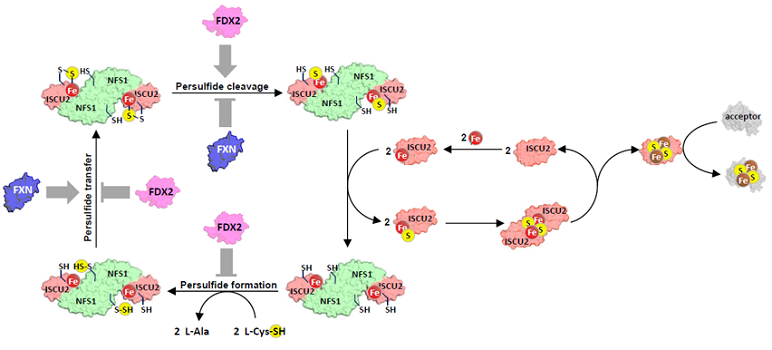
Regulation of Fe–S cluster biosynthesis through antagonistic effects of frataxin (Fxn) and ferredoxin-2 (Fdx2).
© Want et al., 2025, Nature
By providing, for the first time, a complete in vitro description of the assembly steps of a [2Fe–2S] cluster, the team identified key intermediates such as the [1Fe–1S] precursor and demonstrated the existence of finely tuned cross-regulation between Fxn and Fdx2. This regulation explains the toxicity associated with frataxin overexpression and should accelerate the development of gene therapy approaches for Friedreich's ataxia. These results also suggest that reducing Fdx2 expression could represent a novel therapeutic strategy for FRDA patients.
Joliot/I2BC contact : Benoît D'Autréaux (benoit.dautreaux@cea.fr ; benoit.dautreaux@i2bc.paris-saclay.fr)
- Frataxin is a small protein located in the mitochondrial matrix.
- A team from Harvard Medical School jointly demonstrated that, in the Caenorhabditis elegans model organism, point mutations in Fdx2 impair its interaction with Nfs1 and increase the survival of worms lacking frataxin (Fxn).
- Partner laboratories : Medical University of Gdańsk, Poland; University of Strasbourg, CNRS, IPHC UMR 7178 ; ProFI, FR2048 CNRS CEA, Strasbourg ; ICSN, CNRS, Université Paris-Saclay ; Aix-Marseille University (AMU), CNRS, BIP, Marseille; Université Paris-Cité, BFA UMR 8251