Developing new vaccines
Salmonella and Shigella are responsible for gastrointestinal diseases worldwide and are regularly responsible for outbreaks, particularly in South East Asia and Africa. These outbreaks are all the more problematic because of the emergence of multidrug resistance to antibiotics. The vaccines that have been developed are not commercially available or cover only a limited number of serotypes (subspecies). There is therefore a crucial need to develop new broad-spectrum prophylactic or therapeutic vaccines against both bacterial genera. To this end, researchers at LERI (SPI/DMTS) are interested in the type 3 secretion system that allows Salmonella and Shigella to inject their virulence factors through the plasma membrane of host cells. SipD and IpaD proteins, belonging respectively to the tip of the secretion system of Salmonella and Shigella, are good candidates for this type of therapy. In a previous study (see news of 22 June 2020), LERI researchers showed the induction of cross-protection against Salmonella and Shigella infection using SipD or IpaD as immunogen.
A monoclonal antibody targeting 2 proteins
These encouraging results led the researchers to go further: they succeeded in developing a monoclonal antibody (mAb) directed against a common conserved region of the two proteins, despite a protein sequence identity of around 40%. Their work, in collaboration with a team from SIMoS and published in PLoS Neglected Tropical Diseases, shows that the antibody is relatively effective in prophylaxis against Salmonella and Shigella infections in a mouse model. The authors precisely determined the recognition epitope of this mAb, located in the apical part of the SipD and IpaD proteins. In vitro, they showed that mAb has a weak capacity to inhibit the formation of pores in the host cell membrane, and even promotes it. On the other hand, it at least partially blocks bacterial invasion. These observations confirm that pore formation and bacterial invasion, already identified as carried by two distinct domains of IpaD, are mechanistically dissociated.
Since the region targeted by this mAb is essential for pathogen virulence and is conserved among Salmonella and Shigella bacteria, it would be interesting to consider the development and evaluation of chemical compounds or vaccines targeting this specific region for broad spectrum activity. Given recent advances in the engineering of recombinant monoclonal antibodies and studies on their oral administration, particularly against Salmonella infections, the control of diarrhoeal diseases induced by Salmonella and Shigella through the administration of mAbs alone or in combination with other molecules could become realistic and cost-effective.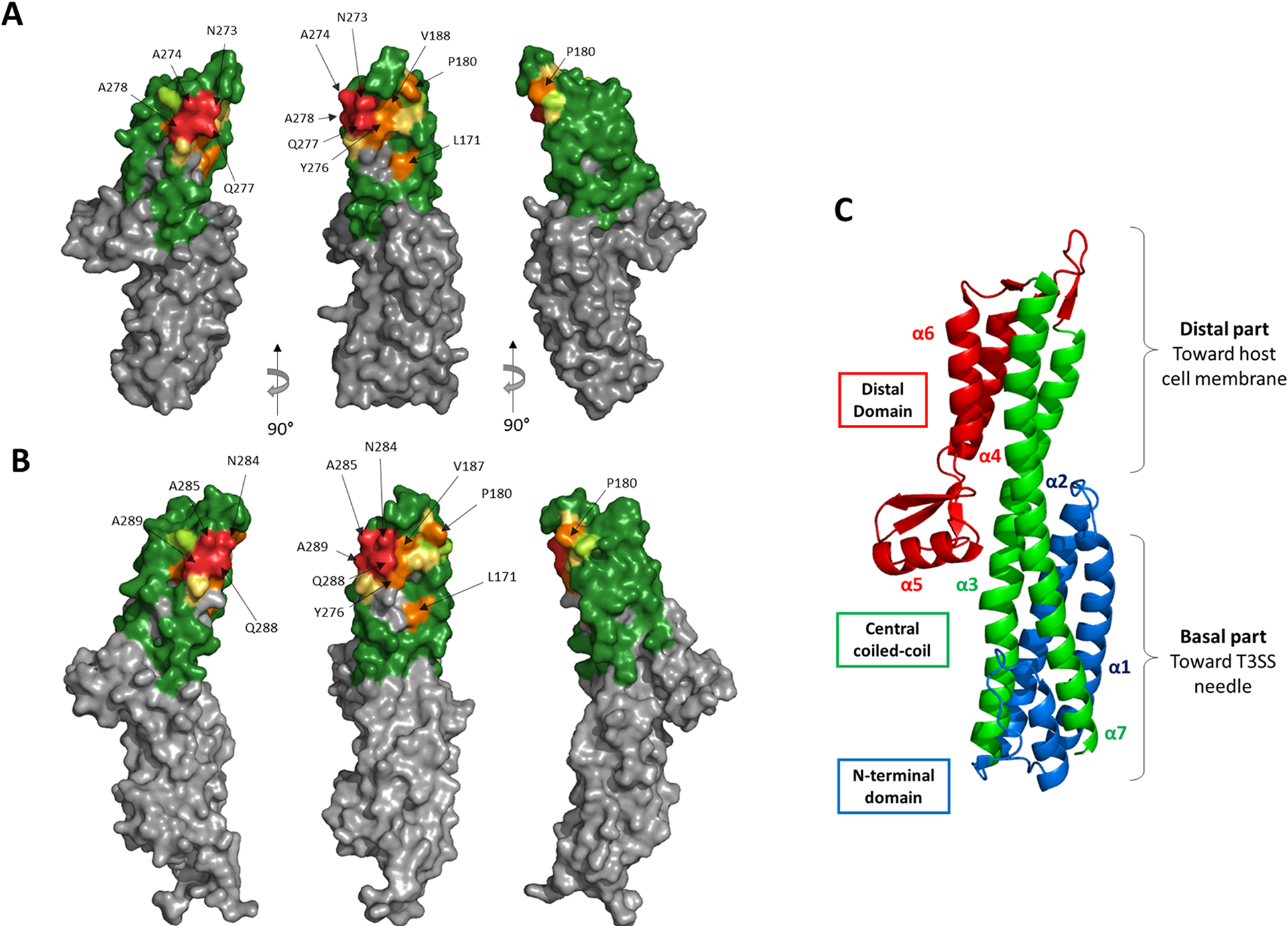
Structural representation of the epitope of mAb IpaD-318 on the antigen IpaD as determined by yeast display.
(A) Residues were colored on the structure of IpaD (PDB #2J0O) by the overall effect color code of Fig 3C, representing the importance of each tested residue in the interaction. Grey residues were not tested. (B) Epitope representation of mAb IpaD-318 on the antigen SipD (PDB #3NZZ). (C) Structural description of IpaD. © Sierocki et al., PLoS Negl. Trop. Dis., 2021
Joliot contact:
Stéphanie Simon (stephanie.simon@cea.fr)