Team leader
Laurent DEVEL
laurent.devel@cea.fr
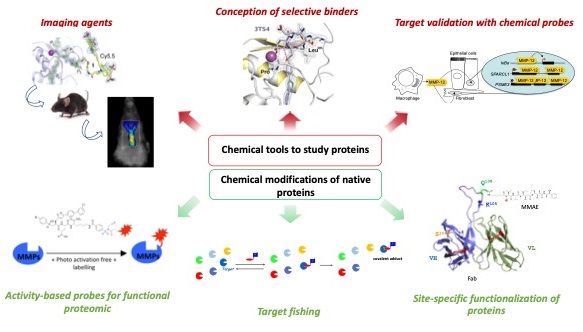
Our team has a long-standing experience in the conception of chemical tools to study enzymes and proteins in systems of variable complexity, including isolated targets, culture cells media and cell lysates as well as living animals. We contributed to identify several highly selective inhibitors of Matrix MetalloProteases (MMPs) (Conception of selective inhibitor: Rouanet-Mehouas et al J. Med. Chem. 2017, Czarny et al., J. Med. Chem. 2013, Devel et al., J. Biol. Chem 2012, Devel et al., J. Biol. Chem 2010, Devel et al., J. Biol. Chem 2006). In preclinical models, the most selective ones notably helped deciphering the essential role of those proteases in atherosclerosis, cancer progression and during viral infection (Target validation with chemical probes: Ella et al., J. Thorac. Cardiovasc. Surg. 2018, Marchant et al, Nat. Med. 2014, Meides et al., Int J Cancer. 2014, Johnson et al., Arterioscler. Thromb. Vasc. Biol. 2011).
Relying on the validation of this inhibitor in living animals, our team also recently designed original imaging agents to specifically detect macrophage elastase (MMP12) in preclinical models of aneurysm and atherosclerosis (Imaging agents: Gona et al., J. Med. Chem 2020, Toczek et al.; J. Med. Chem 2019, Devel et al., Molecules. 2019, Razavian et al., Sci. Report 2016, Bordenave et al, Bioconjugate 2016). With the aim to document the MMPs activation status in different biological processes, we developed several series of reactive chemical probes able to covalently modify those enzymes in complex proteomes and in vivo (Activity-based probes for functional proteomic: Kaminska et al., Angewandte Chem. Int. Ed. 2021, Torkar et al., Bioorg. Med. Chem. Lett 2013, Torkar et al., ChemBioChem 2012, Bregant et al., J. Proteomic Res. 2009, David et al., Angewandte Chem. Int. Ed. 2007). In the same vein, we are currently developing targeted proteomic approaches that combine ligand-directed chemistry and derivatization strategies with a mass tag (Sejalon-Cipolla et al., Trends in Analytical Chemistry 2021). These approaches are also used in “target fishing” projects to determine the privileged protein targets of biologically active compounds.
In parallel, we explore new approaches enabling the site-specific functionalization of proteins. In this field, our recent achievements found applications in the dual radiolabeling of antibody-drug conjugates (ADCs), whose in vivo fate of both the cytotoxic payload and the protein vector can be sensitively and accurately determined by ex vivo digital imaging. Our approach completes the very restricted set of bioanalytical methods available for the detection and quantification of ADCs in various biological matrices (Cahuzac et al., Pharmaceuticals 2020).