The brain is extraordinarily well protected from its environment. For example, compared to the other organs, exchanges between the blood and the brain are strictly regulated and limited. The vascularly-situated mechanism in charge of this protection is called the blood-brain barrier (BBB), and it is vital, notably because it protects the brain from infections. However, the BBB also makes it difficult to deliver sufficient quantities of neurological therapeutic agents. A technique already used in diagnostic echography, microbubble ultrasound, may be a way to resolve this problem. When stimulated by pulsed ultrasounds, microbubbles begin oscillating and induce mechanical stress on capillary walls, resulting in turn in a transitory increase in permeability. Thus, combining trans-cranial, low-frequency ultrasound and intravenously-injected microbubbles may enable a controlled, localized and efficacious delivery of therapeutic drugs to the brain. This completely noninvasive intervention would last only a few minutes and MRI could precisely guide microbubble injection and the ultrasound beam. There are however several challenges to overcome, particularly the potential deleterious effects of the focused ultrasound waves and the inability to guarantee efficacious delivery of the molecule.
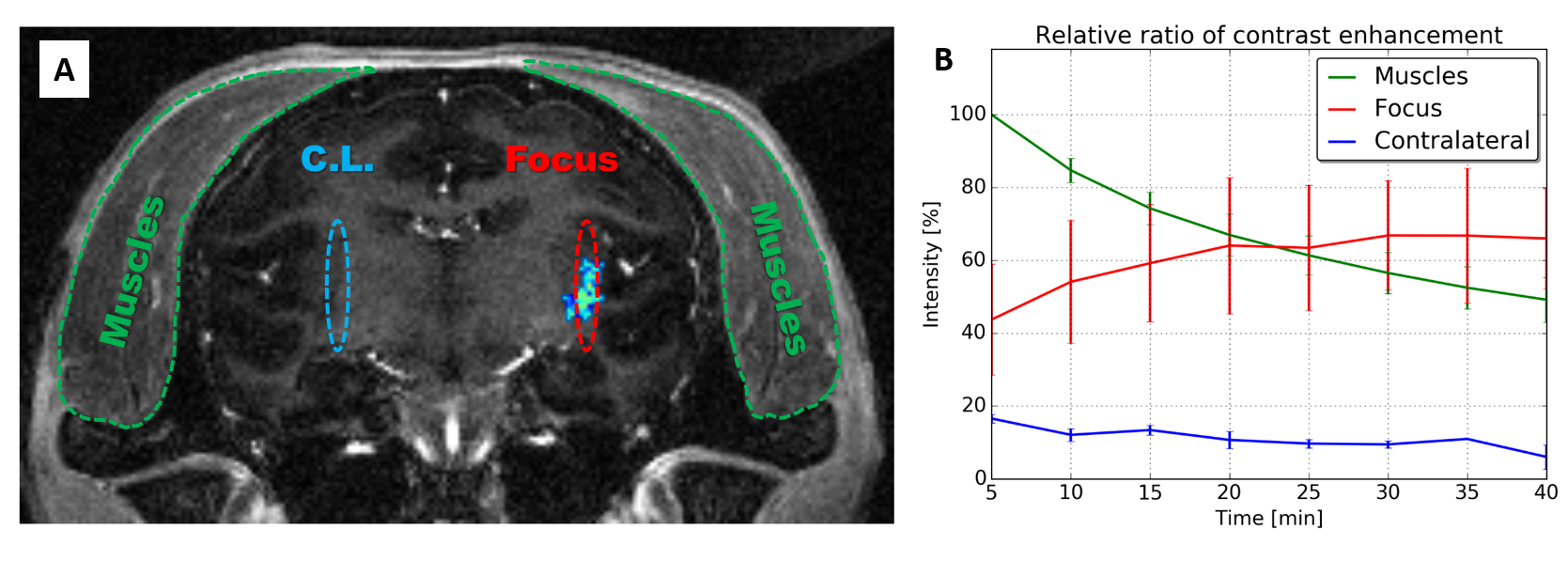
Toward resolving these difficulties, researchers from NeuroSpin and MIRCen have developed an innovative system that allows for the close control of acoustic pressure parameters. Amagnetic and sufficiently compact, the system enables the dynamic adjustment of that pressure via passive cavitation detection, an essential element for ensuring the efficacy, reproducibility and especially safety of the protocol. In a study on non-human primates and using contrast-enhanced MRI, the teams demonstrated a six-fold increase in the amount of the contrast agent crossing the BBB (compared to that normally observed).
This experimental system will serve initially to develop new therapeutic approaches for neurodegenerative diseases. Looking further on, it could evolve into a more complex medical system optimized for humans. Thus, in the long term, the system may greatly increase the number of therapeutics deliverable to the brain in an efficacious and localized manner: small chemotherapy molecules, therapeutic antibodies, gene therapy vectors, etc. This expansion in useable therapeutics could bring great benefit to numerous pathologies, brain cancers (glioblastomas) first but also neurodegenerative, immune and psychiatric disorders thereafter.
A financially supported innovative project
The work presented here benefits from the support of the Technologies for Health program of the CEA, the European Regional Development Fund (ERDF) of the European Union, the scientific interest group IBiSA, and the national infrastructure NeurATRIS (www.neuratris.com). The postdoctoral fellowship for this project and the majority of the experimental systems were financed directly by MIRCen.
A project for the development of this system specifically for humans was recently financed by the Technologies for Health program of the National Research Agency (ANR 3BOPUS) with the additional support of the Medicen competitiveness cluster (http://www.medicen.org/) and Alsace BioValley (http://www.alsace-biovalley.com/fr/).