DNA methylation is an epigenetic marker associated with gene regulation. The process plays key roles in a number of biological processes, including embryogenesis, aging, carcinogenesis, and immune response to infectious diseases.
In mammals, DNA methylation manifests as the addition of a methyl group (CH₃) to cytosine, one of the four nucleobases of DNA. That reaction is catalyzed by two large classes of DNA methyltransferases.
Methylation usually occurs at CpG sites (C representing cytosine, G guanine, and p the phosphate linking them) because of their symmetric nature. There are several enzymatic, immunological, or chemical techniques that can be deployed via microarrays or high throughput sequencing to perform highly detailed genome-wide analyses.
The chemical characterization of cytosine methylation status involves the use of sodium bisulfite, which converts unmethylated cytosine to uracil, but leaves methylated cytosines unaffected. This converted DNA can then be hybridized on microarrays. In the setting of large-cohort pangenomics studies, these microarrays can assay the genomes of a number of samples in parallel and for a reasonable cost. The Infinium Human Methylation450 BeadChip and the Methylation EPIC BeadChip are two of the most popular microarrays currently available. Both are manufactured by Illumina, an American specialist in DNA sequencing whose European Solutions Center is located at Genopole. Those microarrays enable the determination of methylation status for several hundreds of thousands of CpG sites throughout the human genome.
Non-human simian primates (NHPs), because of their phylogenic proximity to humans, are the gold-standard models in biomedical research. Currently however, there are few tools specifically designed for simian genome-wide analyses.
It is this state of affairs that led the CNRGH's Epigenetic and Environment Laboratory (LEE), in partnership with IDMIT and the Institut Pasteur's HIV Inflammation and Persistence Unit, to carry out two studies, one assessing the Infinium Human Methylation450 and Methylation EPIC BeadChips for methylation analysis in NHPs and another presenting an example of their application in simian immunodeficiency virus (SIV) models.
The first study, published in Epigenomics, evaluated the performance of the two microarrays' probes¹, designed originally to assay human DNA methylation, in two NHPs : Asian rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus sabaeus). This initial study provided a precise re-annotation of the probes to ease high throughput DNA methylation analyses for the two NHP species.
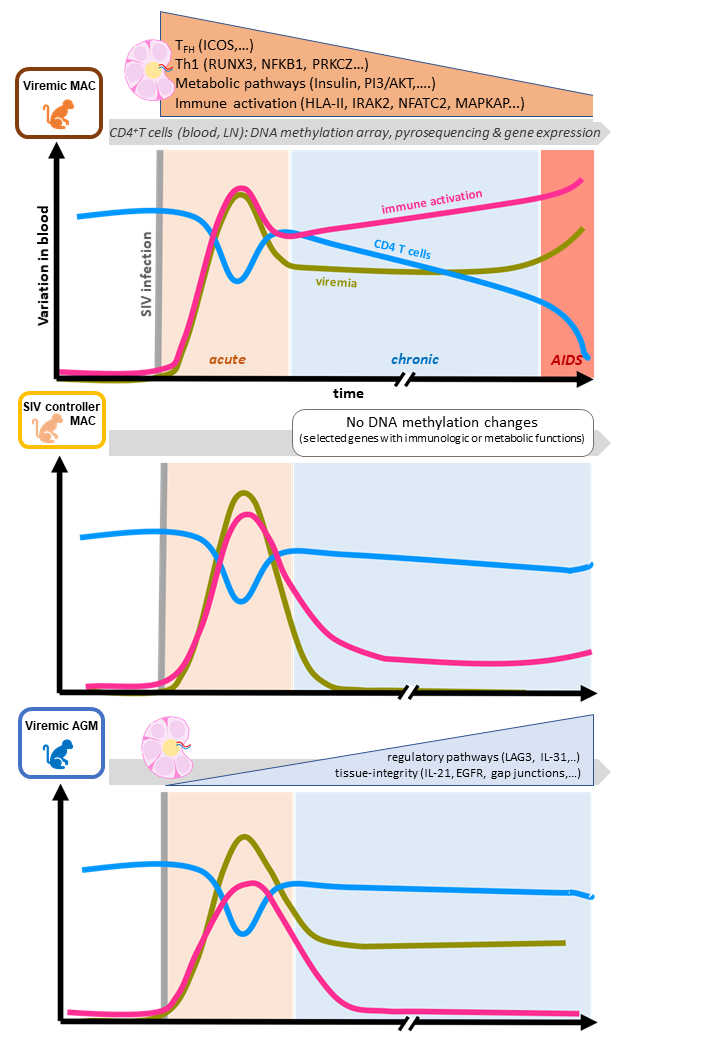
In the second study, published in Clinical Epigenetics, these revamped tools were used to characterize DNA methylation effects in pathogenic SIV infection, using rhesus macaques, and in non-pathogenic SIV infection, using African green monkeys. SIV is the simian retrovirus equivalent to the human immunodeficiency virus (HIV) that causes AIDS in humans. SIV however is a multi-strain virus that exclusively infects about 40 NHPs.
African NHPs like the African green monkey are natural SIV reservoirs. They show high SIV loads in the blood and gut but do not present chronic immune activation or progress toward symptomatic, clinically-detectable disease. They do have an acute immune response in reaction to the primary SIV infection, but thereafter down-regulate it. That immune activation control is maintained throughout the monkey's life, suggesting some underlying, active, efficacious and durable mechanism. Furthermore, in contrast to humans or rhesus macaques, natural reservoirs like the African green monkeys maintain a normal number of CD4+ T cells².
Thus, in the second study, the LEE team and its partners sought to determine whether specific epigenetic modifications play a role in maintaining that long-term immune control. Toward that goal, they used the re-annotated Infinium Human Methylation450 BeadChip microarray to analyze and compare DNA methylation modifications (targeting Blood and lymph node³ CD4+ cells specifically) at several time points in cohorts of SIV-infected African green monkeys and rhesus macaques.
Post-SIV-infection DNA methylation modifications were more pronounced in lymph nodes than in blood and presented as early as acute infection.
After pathogenic SIV infection in Asian rhesus macaques (that develop clinical symptoms), the team observed an increase in methylation among genes involved in the pro-inflammatory Th1 signaling pathway. In contrast, non-pathogenic infection in African green monkeys caused the methylation of genes coding for proteins that in turn contribute to moderate or lessen inflammatory responses and maintain tissue integrity in that model. Furthermore, the pathogenic infection in Asian rhesus macaques provoked DNA methylation changes associated with functional changes in metabolism and immune activation within lymph nodes.
The use of the Infinium Human Methylation450 BeadChip system in this work led to the identification of key genes associated with specific tissues and differentially methylated as early as primary infection that may contribute to the persistence of metabolic disorders and inflammation in simian models of pathogenic infection.
1: A DNA microarray is a rigid support to which short sequences of DNA are attached. Those short sequences are called probes. They are a type of synthetic oligonucleotide. The probes are developed to be specific to a unique target gene. The nucleic acids under study are put into contact with the DNA microarray and its probes, which will hybridize with the target gene.
2: CD4+ T cells, also known as T helper cells, are immune system lymphocytes that, when stimulated, proliferate to direct and activate other immune cells for the elimination of a pathogenic agent. CD4+ T cells are the primary target of the human immunodeficiency virus (HIV), the causative agent of AIDS. Once HIV has gained access to those cells, it multiplies intensely during the acute phase of the infection.
3: Lymph nodes become the main reservoirs of active virus during asymptomatic disease.