Lung transplantation remains limited by the frequent occurrence of chronic lung allograft dysfunction (CLAD), which is the leading cause of mid- and long-term morbidity and mortality. Despite advances in immunosuppressive therapies, no validated non-invasive biomarker currently allows early prediction of which patients will develop this complication. Identifying reliable biological indicators therefore represents a major clinical challenge.
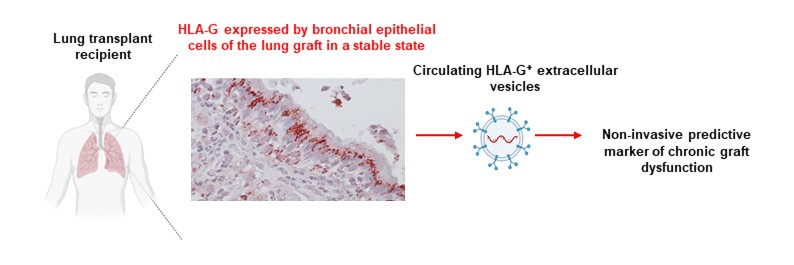
The HLA-G molecule is well known for its immunomodulatory properties and its association with states of immune tolerance. While HLA-G expression within the lung graft has already been correlated with improved graft acceptance, measurements of soluble plasma HLA-G have proven to be poorly informative. The authors therefore hypothesized that the vesicular form of HLA-G, transported by circulating extracellular vesicles, could provide a more accurate reflection of the graft’s immunological status.
@ N. Rouas-Freiss / CEA
The study is based on the analysis of 78 lung transplant recipients from the national COLT cohort, all of whom were clinically stable during the first year after transplantation. Plasma levels of extracellular vesicle–associated HLA-G (HLA-GEV) were measured at 6 and 12 months post-transplantation and then correlated with the subsequent development of the most common CLAD phenotype, bronchiolitis obliterans syndrome (BOS), as well as with three-year graft survival.
The results show that patients who subsequently develop CLAD, particularly BOS, display a significant decrease in HLA-GEV levels at 12 months after transplantation. Conversely, high HLA-GEV levels at this time point are associated with a higher likelihood of sustained stable lung function and improved graft survival. This association remains significant after adjustment for major clinical and immunological factors, making HLA-GEV an independent predictive factor.
The study also highlights the value of dynamic monitoring of this biomarker: a decrease in HLA-GEV levels between 6 and 12 months is specifically observed in patients who go on to develop BOS, suggesting that this approach could enable even earlier detection of chronic rejection risk.
Overall, these findings support the concept that HLA-G bearing extracellular vesicles constitute a true “liquid biopsy” of the lung graft, reflecting mechanisms of immune tolerance or breakdown of tolerance. They pave the way for finer stratification of lung transplant recipients and for personalized adaptation of follow-up and therapeutic strategies.
Contact : Nathalie Rouas-Freiss ; Joël Lemaoult