This topic focuses on the mechanisms of peptide self-assembly and of thestructures of the assemblies. We study the self-assembly properties of therapeutic oligopeptides with a length of 8 to 14 amino-acids. We have two goals: i) understanding and characterizing as finely as possible the physical and physico-chemical rules guiding self-assembly of these molecules and ii) findnovel formulations for therapeutic peptides. This second goal is pursued within "Archi-Pex", a multipartite lab involving three groups from three French cities, two academic groups (Franck Artzner, Institute of Physics . Rennes, and Maité Paternostre, I2BC) and one industry group (Joel Richard, IPSEN-Pharma).
Our basic studies on structures formed by two classes of natural peptidic hormonesand their chemical derivatives and on the mechanisms by which those structures self-assemble have led to many first-rate publications as well as three patents on those peptides' formulations filed together with IPSEN-Pharma.
Structure of peptide nanotubes
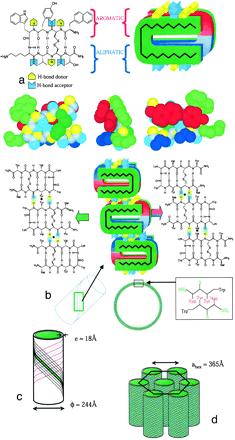
The controlled self-assembly of complex molecules into well-defined hierarchical structures is a promising route for fabricating nanostructures. These nanoscale structures can be realized by naturally occurring proteins such as tobacco mosaic virus, capsid proteins, tubulin, actin, etc. Here, we report a simple alternative method based onself-assembling nanotubes formed by a synthetic therapeutic octapeptide, Lanreotidein water. We used a multidisciplinary approach involving optical and electron microscopies, vibrational spectroscopies, and small and wide angle x-ray scattering to elucidate the hierarchy of structures exhibited by this system. The results revealed the hexagonal packing of nanotubes, and high degree of monodispersity in the tube diameter (244 Å) and wall thickness (18 Å). Moreover, the diameter is tunable by suitable modifications in the molecular structure. The self-assembly of the nanotubes occurs through the association of -sheets driven by amphiphilicity and a systematic aromaticaliphatic side chain segregation. This original and simple system is a unique example for the study of complex self-assembling processes generated by de novo molecules or amyloid peptides.
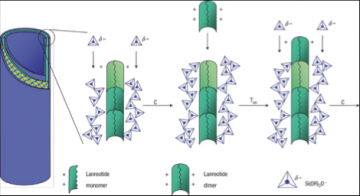
Mechanism of formation of lanreotide nanotubes
Nanofabrication by molecular self-assembly involves the design of molecules and self-assembly strategies so that shape and chemical complementarities drive the units to organize spontaneously into the desired structures. The power of self-assembly makes it the ubiquitous strategy of living organized matter and provides apowerful tool to chemists. However, a challenging issue in the self-assembly of complex supramolecular structures is to understand how kinetically efficient pathways emerge from the multitude of possible transition states and routes. Unfortunately, very few systems provide an intelligible structure and formation mechanism on which new models can be developed. Here, we elucidate the molecular and supramolecular self-assembly mechanism of synthetic octapeptide into nanotubes in equilibrium conditions. Their complex hierarchical self-assembly has recently been described at the mesoscopic level, and we show now that this system uniquely exhibits three assembly stages and three intermediates: (i) a peptide dimer is evidenced by both analytical centrifugation and NMR translational diffusion experiments; (ii) an open ribbon and (iii) an unstable helical ribbon are both visualized by transmission electron microscopy and characterized by small angle X-ray scattering. Interestingly, the structural features of two stable intermediates are related to the final nanotube organization as they set, respectively, the nanotube wall thickness and the final wall curvature radius. We propose that a specific self-assembly pathway is selected by the existence of such preorganized and stable intermediates so that a unique final molecular organization is kinetically favored. Our findings suggests that the rational design of oligopeptides can encode both molecular- and macro-scale morphological characteristics of their higher order assemblies, thus opening the way to ultrahigh resolution peptide scaffold engineering.
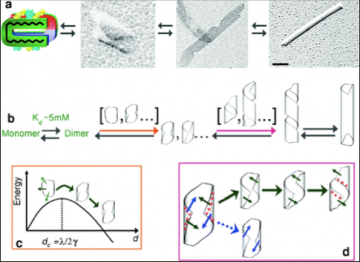
From single molecule to control of assembly size
Supramolecular self-assembly is an attractive pathway for bottom-up synthesis of novel nanomaterials. In particular, this approach allows the spontaneous formation of structures of well-defined shapes and monodisperse characteristic sizes. Because nanotechnology mainly relies on size-dependent physical phenomena, the control of monodispersity is required, but the possibility of tuning the size is also essential. For self-assembling systems, shape, size, and monodispersity are mainly settled by the chemical structure of the building block. Attempts to change the size notably by chemical modification usually end up with the loss of self-assembly. Here, we generated a library of 17 peptides forming nanotubes of monodisperse diameter ranging from 10 to 36 nm. A structural model taking into account close contacts explains how a modification of a few Å of a single aromatic residue induces a fourfold increase in nanotube diameter. The application of such a strategy is demonstrated by the formation of silica nanotubes of various diameters.
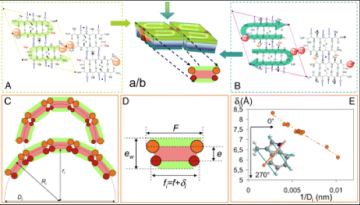
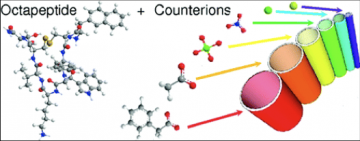
Influence of counter-ions on peptide self-assembly
Among noncovalent forces, electrostatic ones are the strongest and possess a rather long-range action. For these reasons, charges and counterions play a prominent role in selfassembly processes in water and therefore in many biological systems. However, the complexity of the biological media often hinders a detailed understanding of all the electrostatic-related events. In this context, we have studied the role of charges and counterions in the self-assembly of lanreotide, a cationic octapeptide. This peptide spontaneously forms monodisperse nanotubes (NTs) above a critical concentration when solubilized in pure water. Free from any screening buffer, we assessed the interactions between the different peptide oligomers and counterions in solutions, above and below the critical assembly concentration. Our results provide explanations for the selection of a dimeric building block instead of a monomeric one. Indeed, the apparent charge of the dimers is lower than that of the monomers because of strong chemisorption. This phenomenon has two consequences: (i) the dimer−dimer interaction is less repulsive than the monomer−monomer one and (ii) the lowered charge of the dimeric building block weakens the electrostatic repulsion from the positively charged NT walls. Moreover, additional counterion condensation (physisorption) occurs on the NT wall. We furthermore show that the counterions interacting with the NTs play a structural role as they tune the NTs diameter. We demonstrate by a simple model that counterions adsorption sites located on the inner face of the NT walls are responsible for this size control.
Use of lanreotide nanotubes as templates to form double-walled glass nanotubes.
Diatoms, shells, bones and teeth are exquisite examples of well-defined structures, arranged from nanometre to macroscopic length scale, produced by natural biomineralization using organic templates to control the growth of the inorganic phase. Although strategies mimicking Nature have partially succeeded insynthesizing human-designed bio-inorganic composite materials, our limited understanding of fundamental mechanisms has so far kept the level of hierarchical complexity found in biological organisms out of the chemists' reach. In this letter, we report on the synthesis of unprecedented double-walled silica nanotubes with monodisperse diameters that self-organize into highly ordered centimetre-sized fibres. A unique synergistic growth mechanism is elucidated by the combination of light and electron microscopy, synchrotron X-ray diffuse scattering and Raman spectroscopy. Following this growth mechanism, macroscopic bundles of nanotubules result from the kinetic cross-coupling of two molecular processes: adynamical supramolecular self-assembly and a stabilizing silica mineralization.The feedback actions between the template growth and the inorganic deposition are driven by a mutual electrostatic neutralization. This 'dynamical template' concept can be further generalized as a rational preparation scheme for materials with well-defined multiscale architectures and also as a fundamental mechanism for growth processes in biological systems.